Positron emission tomography molecular imaging for drug development
- PMID: 21838787
- PMCID: PMC3269576
- DOI: 10.1111/j.1365-2125.2011.04085.x
Positron emission tomography molecular imaging for drug development
Abstract
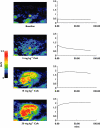
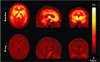
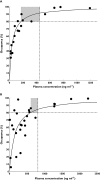
Human in vivo molecular imaging with positron emission tomography (PET) enables a new kind of 'precision pharmacology', able to address questions central to drug development. Biodistribution studies with drug molecules carrying positron-emitting radioisotopes can test whether a new chemical entity reaches a target tissue compartment (such as the brain) in sufficient amounts to be pharmacologically active. Competition studies, using a radioligand that binds to the target of therapeutic interest with adequate specificity, enable direct assessment of the relationship between drug plasma concentration and target occupancy. Tailored radiotracers can be used to measure relative rates of biological processes, while radioligands specific for tissue markers expected to change with treatment can provide specific pharmacodynamic information. Integrated application of PET and magnetic resonance imaging (MRI) methods allows molecular interactions to be related directly to anatomical or physiological changes in a tissue. Applications of imaging in early drug development can suggest approaches to patient stratification for a personalized medicine able to deliver higher value from a drug after approval. Although imaging experimental medicine adds complexity to early drug development and costs per patient are high, appropriate use can increase returns on R and D investment by improving early decision making to reduce new drug attrition in later stages. We urge that the potential value of a translational molecular imaging strategy be considered routinely and at the earliest stages of new drug development.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures

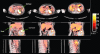
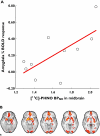

References
-
- Bergstrom M, Grahnen A, Langstrom B. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. Eur J Clin Pharmacol. 2003;59:357–66. - PubMed
-
- Summerfield SG, Stevens AJ, Cutler L, del Carmen Osuna M, Hammond B, Tang SP, Hersey A, Spalding DJ, Jeffrey P. Improving the in vitro prediction of in vivo central nervous system penetration: integrating permeability, P-glycoprotein efflux, and free fractions in blood and brain. J Pharmacol Exp Ther. 2006;316:1282–90. - PubMed
-
- Gunn RN, Summerfield SS, Salinas C, Read KR, Searle G, Ruffo AD, Parker C, Stevens AJ, Bonasera T, Jeffrey PM, Laruelle MA. Combining PET and equilibrium dialysis to assess blood-brain barrier transport. J Cereb Blood Flow Metab. 2007;27(Suppl. 1)
-
- Slifstein M, Laruelle M. Models and methods for derivation of in vivoneuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 2001;28:595–608. - PubMed
-
- Passchier J, Lawrie KWM, Bender D, Fellows I, Gee AD. [11C] Loperamide as highly sensitive PET probe for measuring changes in P-glycoprotein functionality. J Labelled Comp Radiopharm. 2003;46:S94.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

