Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial
- PMID: 21838909
- PMCID: PMC3171378
- DOI: 10.1186/1475-2875-10-237
Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial
Abstract
Background: The use of artemisinin-based combination therapy (ACT) is currently recommended for treating uncomplicated malaria. The objective was to assess the efficacy and safety of repeated administrations of two fixed-dose presentations of ACT--artesunate plus amodiaquine (ASAQ) and artemether-lumefantrine (AL)--in subsequent episodes of Plasmodium falciparum malaria.
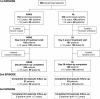
Methods: A randomized comparative study was conducted in a rural community of central Senegal from August 2007 to January 2009. Children and adults with uncomplicated P. falciparum malaria were randomized to receive open-label ASAQ once daily or AL twice daily for three days. Drug doses were given according to body weight. Treatments for first episodes were supervised. For subsequent episodes, only the first intake of study drug was supervised. ECGs and audiograms were performed in patients ≥ 12 years of age. Primary outcome was adequate clinical and parasitological response rate (ACPR) after polymerase chain reaction (PCR) correction on day 28 for the first episode.
Results: A total of 366 patients were enrolled in the two groups (ASAQ 184, AL 182) and followed up during two malaria transmission seasons. In the intent-to-treat population, PCR-corrected ACPRs at day 28 for the first episode were 98.4% and 96.2%, respectively, in the ASAQ and AL groups. For the per-protocol population (ASAQ 183, AL 182), PCR-corrected ACPRs at day 28 for the first episode were 98.9% and 96.7%, respectively. A 100% ACPR rate was obtained at day 28 in the 60 and four patients, respectively, who experienced second and third episodes. Treatment-related adverse events were reported in 11.7% of the patients, without significant differences between the two groups. A better improvement of haemoglobin at day 28 was noted in the ASAQ versus the AL group (12.2 versus 11.8 g/dL; p = 0.03). No sign of ototoxicity was demonstrated. A prolongation of the QTc interval was observed in both groups during treatment with no clinical consequence.
Conclusions: Study results confirmed the satisfactory efficacy and safety profile of ASAQ and AL. Moreover, in patients who were treated at least twice, repeated administration of ASAQ or AL did not identify any major safety issues.
Trial registration: ClinicalTrials.gov identifier NCT00540410.
Figures

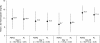
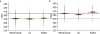
References
-
- World Health Organization. Fact sheet: malaria No. 94 WHO, Geneva 2010. http://www.who.int/mediacentre/factsheets/fs094/en/
-
- Roll Back Malaria Partnership. Roll Back Malaria. Progress and impact series: focus on Senegal. http://www.rbm.who.int/ProgressImpactSeries/report4
-
- World Health Organization. Antimalarial drug combination therapy: Report of a WHO Technical Consultation. WHO Press: Geneva; 2001.
-
- World Health Organization. Guidelines for the treatment of malaria. WHO Press: Geneva; 2006.
-
- World Health Organization. Guidelines for the treatment of malaria. Second. WHO Press, Geneva; 2010.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

