Ethics and animal numbers: informal analyses, uncertain sample sizes, inefficient replications, and type I errors
- PMID: 21838970
- PMCID: PMC3148647
Ethics and animal numbers: informal analyses, uncertain sample sizes, inefficient replications, and type I errors
Abstract
To obtain approval for the use vertebrate animals in research, an investigator must assure an ethics committee that the proposed number of animals is the minimum necessary to achieve a scientific goal. How does an investigator make that assurance? A power analysis is most accurate when the outcome is known before the study, which it rarely is. A 'pilot study' is appropriate only when the number of animals used is a tiny fraction of the numbers that will be invested in the main study because the data for the pilot animals cannot legitimately be used again in the main study without increasing the rate of type I errors (false discovery). Traditional significance testing requires the investigator to determine the final sample size before any data are collected and then to delay analysis of any of the data until all of the data are final. An investigator often learns at that point either that the sample size was larger than necessary or too small to achieve significance. Subjects cannot be added at this point in the study without increasing type I errors. In addition, journal reviewers may require more replications in quantitative studies than are truly necessary. Sequential stopping rules used with traditional significance tests allow incremental accumulation of data on a biomedical research problem so that significance, replicability, and use of a minimal number of animals can be assured without increasing type I errors.
Copyright 2011 by the American Association for Laboratory Animal Science
Figures
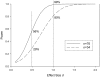
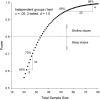

References
-
- Botella J, Ximenez C, Revuelta J, Suero M. 2006. Optimization of sample size in controlled experiments: the CLAST rule. Behav Res Methods 38:65–76 - PubMed
-
- Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale (NJ): Erlbaum
-
- Cumming G. 2005. Understanding the average probability of replication: comment on Killeen (2005). Psychol Sci 16:1002–1004 - PubMed
-
- Desbiens NA. 2003. A novel use for the word ‘trend’ in the clinical trial literature. Am J Med Sci 326:61–65 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
