Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway
- PMID: 21839714
- PMCID: PMC3181349
- DOI: 10.1016/j.ajpath.2011.06.033
Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway
Abstract
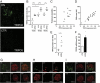
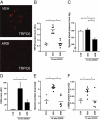
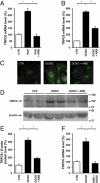
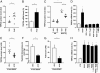
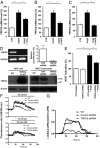
The transient receptor potential channel C6 (TRPC6) is a slit diaphragm-associated protein in podocytes involved in regulating glomerular filter function. Gain-of-function mutations in TRPC6 cause hereditary focal segmental glomerulosclerosis (FSGS), and several human acquired proteinuric diseases show increased glomerular TRPC6 expression. Angiotensin II (AngII) is a key contributor to glomerular disease and may regulate TRPC6 expression in nonrenal cells. We demonstrate that AngII regulates TRPC6 mRNA and protein levels in cultured podocytes and that AngII infusion enhances glomerular TRPC6 expression in vivo. In animal models for human FSGS (doxorubicin nephropathy) and increased renin-angiotensin system activity (Ren2 transgenic rats), glomerular TRPC6 expression was increased in an AngII-dependent manner. TRPC6 expression correlated with glomerular damage markers and glomerulosclerosis. We show that the regulation of TRPC6 expression by AngII and doxorubicin requires TRPC6-mediated Ca(2+) influx and the activation of the Ca(2+)-dependent protein phosphatase calcineurin and its substrate nuclear factor of activated T cells (NFAT). Accordingly, calcineurin inhibition by cyclosporine decreased TRPC6 expression and reduced proteinuria in doxorubicin nephropathy, whereas podocyte-specific inducible expression of a constitutively active NFAT mutant increased TRPC6 expression and induced severe proteinuria. Our findings demonstrate that the deleterious effects of AngII on podocytes and its pathogenic role in glomerular disease involve enhanced TRPC6 expression via a calcineurin/NFAT positive feedback signaling pathway.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Faul C., Asanuma K., Yanagida-Asanuma E., Kim K., Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. - PubMed
-
- Reiser J., Polu K.R., Moller C.C., Kenlan P., Altintas M.M., Wei C., Faul C., Herbert S., Villegas I., Avila-Casado C., McGee M., Sugimoto H., Brown D., Kalluri R., Mundel P., Smith P.L., Clapham D.E., Pollak M.R. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. - PMC - PubMed
-
- Winn M.P., Conlon P.J., Lynn K.L., Farrington M.K., Creazzo T., Hawkins A.F., Daskalakis N., Kwan S.Y., Ebersviller S., Burchette J.L., Pericak-Vance M.A., Howell D.N., Vance J.M., Rosenberg P.B. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. - PubMed
-
- Moller C.C., Wei C., Altintas M.M., Li J., Greka A., Ohse T., Pippin J.W., Rastaldi M.P., Wawersik S., Schiavi S., Henger A., Kretzler M., Shankland S.J., Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

