Acute and chronic regulation of aldosterone production
- PMID: 21839803
- PMCID: PMC3253327
- DOI: 10.1016/j.mce.2011.07.034
Acute and chronic regulation of aldosterone production
Abstract
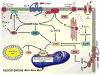
Aldosterone is the major mineralocorticoid synthesized by the adrenal and plays an important role in the regulation of systemic blood pressure through the absorption of sodium and water. Aldosterone production is regulated tightly by selective expression of aldosterone synthase (CYP11B2) in the adrenal outermost zone, the zona glomerulosa. Angiotensin II (Ang II), potassium (K(+)) and adrenocorticotropin (ACTH) are the main physiological agonists which regulate aldosterone secretion. Aldosterone production is regulated within minutes of stimulation (acutely) through increased expression and phosphorylation of the steroidogenic acute regulatory (StAR) protein and over hours to days (chronically) by increased expression of the enzymes involved in the synthesis of aldosterone, particularly CYP11B2. Imbalance in any of these processes may lead to several disorders of aldosterone excess. In this review we attempt to summarize the key molecular events involved in the acute and chronic phases of aldosterone secretion.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures


References
-
- Adler GK, Chen R, Menachery AI, Braley LM, Williams GH. Sodium restriction increases aldosterone biosynthesis by increasing late pathway, but not early pathway, messenger ribonucleic acid levels and enzyme activity in normotensive rats. Endocrinology. 1993;133:2235–40. - PubMed
-
- Allen RG, Carey C, Parker JD, Mortrud MT, Mellon SH, Low MJ. Targeted ablation of pituitary pre-proopiomelanocortin cells by herpes simplex virus-1 thymidine kinase differentially regulates mRNAs encoding the adrenocorticotropin receptor and aldosterone synthase in the mouse adrenal gland. Mol Endocrinol. 1995;9:1005–16. - PubMed
-
- Aptel HB, Burnay MM, Rossier MF, Capponi AM. The role of tyrosine kinases in capacitative calcium influx-mediated aldosterone production in bovine adrenal zona glomerulosa cells. J Endocrinol. 1999;163:131–8. - PubMed
-
- Aptel HB, Johnson EI, Vallotton MB, Rossier MF, Capponi AM. Demonstration of an angiotensin II-induced negative feedback effect on aldosterone synthesis in isolated rat adrenal glomerulosa cells. Mol Cell Endocrinol. 1996;119:105–111. - PubMed
-
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JFI. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem. 1997;272:32656–32662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

