Modafinil for the treatment of methamphetamine dependence
- PMID: 21840138
- PMCID: PMC3227772
- DOI: 10.1016/j.drugalcdep.2011.07.007
Modafinil for the treatment of methamphetamine dependence
Abstract
Aim: Modafinil was tested for efficacy in decreasing use in methamphetamine-dependent participants, compared to placebo.
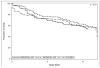
Methods: This was a randomized, double-blind, placebo-controlled study, with 12 weeks of treatment and a 4-week follow-up. Eight outpatient substance abuse treatment clinics participated in the study. There were 210 treatment-seekers randomized, who all had a DSM-IV diagnosis of methamphetamine dependence; 68 participants to placebo, 72 to modafinil 200mg, and 70 to modafinil 400mg, taken once daily on awakening. Participants came to the clinic three times per week for assessments, urine drug screens, and group psychotherapy. The primary outcome measure was a methamphetamine non-use week, which required all the week's qualitative urine drug screens to be negative for methamphetamine.
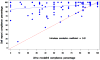
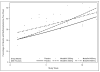
Results: Regression analysis showed no significant difference between either modafinil group (200 or 400mg) or placebo in change in weekly percentage having a methamphetamine non-use week over the 12-week treatment period (p=0.53). Similarly, a number of secondary outcomes did not show significant effects of modafinil. However, an ad-hoc analysis of medication compliance, by urinalysis for modafinil and its metabolite, did find a significant difference in maximum duration of abstinence (23 days vs. 10 days, p=0.003), between those having the top quartile of compliance (>85% of urines were positive for modafinil, N=36), and the lower three quartiles of modafinil 200 and 400mg groups (N=106).
Conclusions: Although these data suggest that modafinil, plus group behavioral therapy, was not effective for decreasing methamphetamine use, the study is probably inconclusive because of inadequate compliance with taking medication.
Published by Elsevier Ireland Ltd.
Conflict of interest statement
Figures


References
-
- Adler LA, Spencer T, Faraone SV, Reimherr FW, Kelsey D, Michelson D, Biederman J. Training raters to assess adult ADHD: reliability of ratings. J Atten Disord. 2005;8:121–126. - PubMed
-
- Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. - PubMed
-
- Cephalon, Inc. Cephalon, Inc.; Frazer, PA: 2007. [accessed 02/04/11]. PROVIGIL® (modafinil) Tablets [C-IV], FDA Approved Labeling - dated August 17, 2007. www.accessdata.fda.gov/drugsatfda_docs/label/2007/020717s020s013s018lbl.pdf.
-
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

