Long-term rescue of a familial hypertrophic cardiomyopathy caused by a mutation in the thin filament protein, tropomyosin, via modulation of a calcium cycling protein
- PMID: 21840315
- PMCID: PMC3221410
- DOI: 10.1016/j.yjmcc.2011.07.026
Long-term rescue of a familial hypertrophic cardiomyopathy caused by a mutation in the thin filament protein, tropomyosin, via modulation of a calcium cycling protein
Abstract
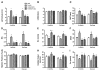
We have recently shown that a temporary increase in sarcoplasmic reticulum (SR) cycling via adenovirus-mediated overexpression of sarcoplasmic reticulum ATPase (SERCA2) transiently improves relaxation and delays hypertrophic remodeling in a familial hypertrophic cardiomyopathy (FHC) caused by a mutation in the thin filament protein, tropomyosin (i.e., α-TmE180G or Tm180). In this study, we sought to permanently alter calcium fluxes via phospholamban (PLN) gene deletion in Tm180 mice in order to sustain long-term improvements in cardiac function and adverse cardiac remodeling/hypertrophy. While similar work has been done in FHCs resulting from mutations in thick myofilament proteins, no one has studied these effects in an FHC resulting from a thin filament protein mutation. Tm180 transgenic (TG) mice were crossbred with PLN knockout (KO) mice and four groups were studied in parallel: 1) non-TG (NTG), 2) Tm180, 3) PLNKO/NTG and 4) PLNKO/Tm180. Tm180 mice exhibit increased heart weight/body weight and hypertrophic gene markers compared to NTG mice, but levels in PLNKO/Tm180 mice were similar to NTG. Tm180 mice also displayed altered function as assessed via in situ pressure-volume analysis and echocardiography at 3-6 months and one year; however, altered function in Tm180 mice was rescued back to NTG levels in PLNKO/Tm180 mice. Collagen deposition, as assessed by Picrosirius Red staining, was increased in Tm180 mice but was similar in NTG and in PLNKO/Tm180 mice. Extracellular signal-regulated kinase (ERK1/2) phosphorylation increased in Tm180 mice while levels in PLNKO/Tm180 mice were similar to NTGs. The present study shows that by modulating SR calcium cycling, we were able to rescue many of the deleterious aspects of FHC caused by a mutation in the thin filament protein, Tm.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

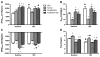

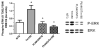
Similar articles
-
Microarray analysis of active cardiac remodeling genes in a familial hypertrophic cardiomyopathy mouse model rescued by a phospholamban knockout.Physiol Genomics. 2013 Sep 3;45(17):764-73. doi: 10.1152/physiolgenomics.00023.2013. Epub 2013 Jun 25. Physiol Genomics. 2013. PMID: 23800848 Free PMC article.
-
Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins.Circ Cardiovasc Genet. 2014 Apr;7(2):132-143. doi: 10.1161/CIRCGENETICS.113.000324. Epub 2014 Feb 28. Circ Cardiovasc Genet. 2014. PMID: 24585742 Free PMC article.
-
Rescue of tropomyosin-induced familial hypertrophic cardiomyopathy mice by transgenesis.Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H949-58. doi: 10.1152/ajpheart.01341.2006. Epub 2007 Apr 6. Am J Physiol Heart Circ Physiol. 2007. PMID: 17416600
-
Alpha-tropomyosin mutations in inherited cardiomyopathies.J Muscle Res Cell Motil. 2013 Aug;34(3-4):285-94. doi: 10.1007/s10974-013-9358-5. Epub 2013 Sep 5. J Muscle Res Cell Motil. 2013. PMID: 24005378 Review.
-
Cellular and molecular aspects of familial hypertrophic cardiomyopathy caused by mutations in the cardiac troponin I gene.Mol Cell Biochem. 2004 Aug;263(1-2):99-114. doi: 10.1023/B:MCBI.0000041852.42291.aa. Mol Cell Biochem. 2004. PMID: 15524171 Review.
Cited by
-
Conserved Asp-137 is important for both structure and regulatory functions of cardiac α-tropomyosin (α-TM) in a novel transgenic mouse model expressing α-TM-D137L.J Biol Chem. 2013 Jun 7;288(23):16235-16246. doi: 10.1074/jbc.M113.458695. Epub 2013 Apr 22. J Biol Chem. 2013. PMID: 23609439 Free PMC article.
-
p90 ribosomal S6 kinase 3 contributes to cardiac insufficiency in α-tropomyosin Glu180Gly transgenic mice.Am J Physiol Heart Circ Physiol. 2013 Oct 1;305(7):H1010-9. doi: 10.1152/ajpheart.00237.2013. Epub 2013 Aug 2. Am J Physiol Heart Circ Physiol. 2013. PMID: 23913705 Free PMC article.
-
Gene therapy for inherited arrhythmias.Cardiovasc Res. 2020 Jul 15;116(9):1635-1650. doi: 10.1093/cvr/cvaa107. Cardiovasc Res. 2020. PMID: 32321160 Free PMC article. Review.
-
Cardiomyopathy: pathogenesis and therapeutic interventions.MedComm (2020). 2024 Oct 25;5(11):e772. doi: 10.1002/mco2.772. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39465141 Free PMC article. Review.
-
Low-Level Nanog Expression in the Regulation of Quiescent Endothelium.Arterioscler Thromb Vasc Biol. 2020 Sep;40(9):2244-2264. doi: 10.1161/ATVBAHA.120.314875. Epub 2020 Jul 9. Arterioscler Thromb Vasc Biol. 2020. PMID: 32640900 Free PMC article.
References
-
- Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104(4):557–67. - PubMed
-
- Seggewiss H, Blank C, Pfeiffer B, Rigopoulos A. Hypertrophic cardiomyopathy as a cause of sudden death. Herz. 2009;34(4):305–14. - PubMed
-
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287(10):1308–20. - PubMed
-
- Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, et al. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77(5):701–12. - PubMed
-
- Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart failure reviews. 2005;10(3):237–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

