A full range of mouse sinoatrial node AP firing rates requires protein kinase A-dependent calcium signaling
- PMID: 21840316
- PMCID: PMC3184386
- DOI: 10.1016/j.yjmcc.2011.07.028
A full range of mouse sinoatrial node AP firing rates requires protein kinase A-dependent calcium signaling
Abstract
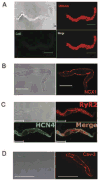
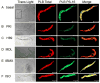
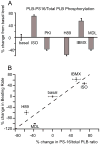
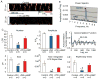
Recent perspectives on sinoatrial nodal cell (SANC)(*) function indicate that spontaneous sarcoplasmic reticulum (SR) Ca(2+) cycling, i.e. an intracellular "Ca(2+) clock," driven by cAMP-mediated, PKA-dependent phosphorylation, interacts with an ensemble of surface membrane electrogenic molecules ("surface membrane clock") to drive SANC normal automaticity. The role of AC-cAMP-PKA-Ca(2+) signaling cascade in mouse, the species most often utilized for genetic manipulations, however, has not been systematically tested. Here we show that Ca(2+) cycling proteins (e.g. RyR2, NCX1, and SERCA2) are abundantly expressed in mouse SAN and that spontaneous, rhythmic SR generated local Ca(2+) releases (LCRs) occur in skinned mouse SANC, clamped at constant physiologic [Ca(2+)]. Mouse SANC also exhibits a high basal level of phospholamban (PLB) phosphorylation at the PKA-dependent site, Serine16. Inhibition of intrinsic PKA activity or inhibition of PDE in SANC, respectively: reduces or increases PLB phosphorylation, and markedly prolongs or reduces the LCR period; and markedly reduces or accelerates SAN spontaneous firing rate. Additionally, the increase in AP firing rate by PKA-dependent phosphorylation by β-adrenergic receptor (β-AR) stimulation requires normal intracellular Ca(2+) cycling, because the β-AR chronotropic effect is markedly blunted when SR Ca(2+) cycling is disrupted. Thus, AC-cAMP-PKA-Ca(2+) signaling cascade is a major mechanism of normal automaticity in mouse SANC.
Published by Elsevier Ltd.
Figures


Similar articles
-
Sarcoplasmic reticulum Ca2+ cycling protein phosphorylation in a physiologic Ca2+ milieu unleashes a high-power, rhythmic Ca2+ clock in ventricular myocytes: relevance to arrhythmias and bio-pacemaker design.J Mol Cell Cardiol. 2014 Jan;66:106-15. doi: 10.1016/j.yjmcc.2013.11.011. Epub 2013 Nov 22. J Mol Cell Cardiol. 2014. PMID: 24274954 Free PMC article.
-
Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand.J Mol Cell Cardiol. 2011 Nov;51(5):740-8. doi: 10.1016/j.yjmcc.2011.07.018. Epub 2011 Jul 28. J Mol Cell Cardiol. 2011. PMID: 21835182 Free PMC article.
-
Cholinergic receptor signaling modulates spontaneous firing of sinoatrial nodal cells via integrated effects on PKA-dependent Ca(2+) cycling and I(KACh).Am J Physiol Heart Circ Physiol. 2009 Sep;297(3):H949-59. doi: 10.1152/ajpheart.01340.2008. Epub 2009 Jun 19. Am J Physiol Heart Circ Physiol. 2009. PMID: 19542482 Free PMC article.
-
Unique Ca2+-Cycling Protein Abundance and Regulation Sustains Local Ca2+ Releases and Spontaneous Firing of Rabbit Sinoatrial Node Cells.Int J Mol Sci. 2018 Jul 25;19(8):2173. doi: 10.3390/ijms19082173. Int J Mol Sci. 2018. PMID: 30044420 Free PMC article. Review.
-
Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick.Ann N Y Acad Sci. 2005 Jun;1047:138-56. doi: 10.1196/annals.1341.013. Ann N Y Acad Sci. 2005. PMID: 16093492 Review.
Cited by
-
Circadian regulation of sinoatrial nodal cell pacemaking function: Dissecting the roles of autonomic control, body temperature, and local circadian rhythmicity.PLoS Comput Biol. 2024 Feb 26;20(2):e1011907. doi: 10.1371/journal.pcbi.1011907. eCollection 2024 Feb. PLoS Comput Biol. 2024. PMID: 38408116 Free PMC article.
-
Dual Activation of Phosphodiesterases 3 and 4 Regulates Basal Spontaneous Beating Rate of Cardiac Pacemaker Cells: Role of Compartmentalization?Front Physiol. 2018 Oct 9;9:1301. doi: 10.3389/fphys.2018.01301. eCollection 2018. Front Physiol. 2018. PMID: 30356755 Free PMC article.
-
New kids on the block: The Popeye domain containing (POPDC) protein family acting as a novel class of cAMP effector proteins in striated muscle.Cell Signal. 2017 Dec;40:156-165. doi: 10.1016/j.cellsig.2017.09.015. Epub 2017 Sep 20. Cell Signal. 2017. PMID: 28939104 Free PMC article. Review.
-
Genetic inhibition of Na+-Ca2+ exchanger current disables fight or flight sinoatrial node activity without affecting resting heart rate.Circ Res. 2013 Jan 18;112(2):309-17. doi: 10.1161/CIRCRESAHA.111.300193. Epub 2012 Nov 27. Circ Res. 2013. PMID: 23192947 Free PMC article.
-
Phosphodiesterase in heart and vessels: from physiology to diseases.Physiol Rev. 2024 Apr 1;104(2):765-834. doi: 10.1152/physrev.00015.2023. Epub 2023 Nov 16. Physiol Rev. 2024. PMID: 37971403 Free PMC article. Review.
References
-
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High Basal Protein Kinase A-Dependent Phosphorylation Drives Rhythmic Internal Ca2+ Store Oscillations and Spontaneous Beating of Cardiac Pacemaker Cells. Circ Res. 2006;98:505–14. - PubMed
-
- Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, et al. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res. 2008;102:761–9. - PubMed
-
- Ludwig A, Herrmann S, Hoesl E, Stieber J. Mouse models for studying pacemaker channel function and sinus node arrhythmia. Prog Biophys Mol Biol. 2010;98:179–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

