The 'active life' of Hsp90 complexes
- PMID: 21840346
- PMCID: PMC3793855
- DOI: 10.1016/j.bbamcr.2011.07.020
The 'active life' of Hsp90 complexes
Abstract
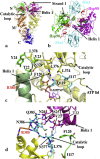
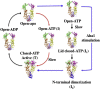
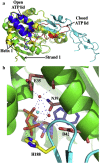
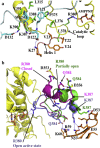
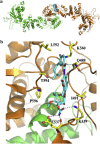
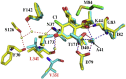
Hsp90 forms a variety of complexes differing both in clientele and co-chaperones. Central to the role of co-chaperones in the formation of Hsp90 complexes is the delivery of client proteins and the regulation of the ATPase activity of Hsp90. Determining the mechanisms by which co-chaperones regulate Hsp90 is essential in understanding the assembly of these complexes and the activation and maturation of Hsp90's clientele. Mechanistically, co-chaperones alter the kinetics of the ATP-coupled conformational changes of Hsp90. The structural changes leading to the formation of a catalytically active unit involve all regions of the Hsp90 dimer. Their complexity has allowed different orthologues of Hsp90 to evolve kinetically in slightly different ways. The interaction of the cytosolic Hsp90 with a variety of co-chaperones lends itself to a complex set of different regulatory mechanisms that modulate Hsp90's conformation and ATPase activity. It also appears that the conformational switches of Hsp90 are not necessarily coupled under all circumstances. Here, I described different co-chaperone complexes and then discuss in detail the mechanisms and role that specific co-chaperones play in this. I will also discuss emerging evidence that post-translational modifications also affect the ATPase activity of Hsp90, and thus complex formation. Finally, I will present evidence showing how Hsp90's active site, although being highly conserved, can be altered to show resistance to drug binding, but still maintain ATP binding and ATPase activity. Such changes are therefore unlikely to significantly alter Hsp90's interactions with client proteins and co-chaperones. This article is part of a Special Issue entitled: Heat Shock Protein 90 (HSP90).
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



References
-
- Nagy P.D., Wang R.Y., Pogany J., Hafren A., Makinen K. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology. 2011;411:374–382. - PubMed
-
- Jakob U., Scheibel T., Bose S., Reinstein J., Buchner J. Assessment of the ATP binding properties of Hsp90. J. Biol. Chem. 1996;271:10035–10041. - PubMed
-
- Smith D.F., Stensgard B.A., Welch W.J., Toft D.O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J. Biol. Chem. 1992;267:1350–1356. - PubMed
-
- Csermely P., Kajtar J., Hollosi M., Jalsovszky G., Holly S., Kahn C.R., Gergely P., Jr., Soti C., Mihaly K., Somogyi J. ATP induces a conformational change of the 90-kDa heat shock protein (Hsp90) J. Biol. Chem. 1993;268:1901–1907. - PubMed
-
- Prodromou C., Roe S.M., O'Brien R., Ladbury J.E., Piper P.W., Pearl L.H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

