Effects of the novel glycopeptide opioid agonist MMP-2200 in preclinical models of Parkinson's disease
- PMID: 21840512
- PMCID: PMC3167001
- DOI: 10.1016/j.brainres.2011.07.038
Effects of the novel glycopeptide opioid agonist MMP-2200 in preclinical models of Parkinson's disease
Abstract
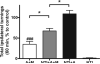
In Parkinson's disease (PD), the consequence of dopaminergic denervation is an imbalance in the activity of the direct and indirect striatofugal pathways, which include potentially important changes in opioid peptide expression and/or activity. The systemic administration of a novel glycosylated opioid peptide MMP-2200 (a.k.a. lactomorphin) was shown to have potent effects in two standard models of PD: 1) amphetamine-induced rotations in the hemi-Parkinsonian 6-hydroxydopamine (6-OHDA)-treated rat and 2) locomotion in the reserpine-treated rat. MMP-2200, an opioid mu and delta receptor agonist, reduced amphetamine-induced rotations in severely-lesioned hemi-Parkinsonian rats; this effect was fully blocked by naloxone, an opioid receptor antagonist. The selective δ-opioid receptor antagonist naltrindole only partially blocked the effect of MMP-2200. MMP-2200 alone did not induce rotations. This effect was also observed in a mild progressive rat 6-OHDA-lesion model. In animals treated with reserpine, profound akinesia was induced that was reversed with apomorphine. There was a prominent overshoot in animals that received apomorphine compared to non-reserpine treated animals, reflecting the well described phenomenon of dopamine supersensitivity indicating that apomorphine not only reversed akinesia but also induced hyper-kinesia. The opioid peptide MMP-2200 blocked the apomorphine-induced hyper-kinesia. This effect of MMP-2200 was prevented by pre-administration of naloxone. MMP-2200 had no effect in preventing the reserpine-induced akinesia, nor did it affect locomotion in control animals. Taken together, the results from these two models are consistent with the glycopeptide opioid agonist MMP-2200 having a potent effect on movements related to dopaminergic hyper-stimulation following striatal dopamine depletion that are best explained by a reduction in the downstream effects of dopamine agonists in these models.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures






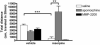


Similar articles
-
The Delta-Specific Opioid Glycopeptide BBI-11008: CNS Penetration and Behavioral Analysis in a Preclinical Model of Levodopa-Induced Dyskinesia.Int J Mol Sci. 2020 Dec 22;22(1):20. doi: 10.3390/ijms22010020. Int J Mol Sci. 2020. PMID: 33374986 Free PMC article.
-
The combination of the opioid glycopeptide MMP-2200 and a NMDA receptor antagonist reduced l-DOPA-induced dyskinesia and MMP-2200 by itself reduced dopamine receptor 2-like agonist-induced dyskinesia.Neuropharmacology. 2018 Oct;141:260-271. doi: 10.1016/j.neuropharm.2018.09.005. Epub 2018 Sep 7. Neuropharmacology. 2018. PMID: 30201210 Free PMC article.
-
In vivo characterization of MMP-2200, a mixed δ/μ opioid agonist, in mice.J Pharmacol Exp Ther. 2011 Mar;336(3):767-78. doi: 10.1124/jpet.110.172866. Epub 2010 Nov 30. J Pharmacol Exp Ther. 2011. PMID: 21118955 Free PMC article.
-
DPI-289, a novel mixed delta opioid agonist / mu opioid antagonist (DAMA), has L-DOPA-sparing potential in Parkinson's disease.Neuropharmacology. 2018 Mar 15;131:116-127. doi: 10.1016/j.neuropharm.2017.11.046. Epub 2017 Nov 29. Neuropharmacology. 2018. PMID: 29197517
-
Mechanisms underlying δ- and μ-opioid receptor agonist-induced increases in extracellular dopamine level in the nucleus accumbens of freely moving rats.J Oral Sci. 2017;59(2):195-200. doi: 10.2334/josnusd.16-0874. J Oral Sci. 2017. PMID: 28637978 Review.
Cited by
-
Neuroprotection or Neurotoxicity of Illicit Drugs on Parkinson's Disease.Life (Basel). 2020 Jun 11;10(6):86. doi: 10.3390/life10060086. Life (Basel). 2020. PMID: 32545328 Free PMC article. Review.
-
Long-term effect of sub-anesthetic ketamine in reducing L-DOPA-induced dyskinesias in a preclinical model.Neurosci Lett. 2016 Jan 26;612:121-125. doi: 10.1016/j.neulet.2015.11.047. Epub 2015 Nov 28. Neurosci Lett. 2016. PMID: 26644333 Free PMC article.
-
The Delta-Specific Opioid Glycopeptide BBI-11008: CNS Penetration and Behavioral Analysis in a Preclinical Model of Levodopa-Induced Dyskinesia.Int J Mol Sci. 2020 Dec 22;22(1):20. doi: 10.3390/ijms22010020. Int J Mol Sci. 2020. PMID: 33374986 Free PMC article.
-
δ-opioid receptor activation protects against Parkinson's disease-related mitochondrial dysfunction by enhancing PINK1/Parkin-dependent mitophagy.Aging (Albany NY). 2020 Nov 10;12(24):25035-25059. doi: 10.18632/aging.103970. Epub 2020 Nov 10. Aging (Albany NY). 2020. PMID: 33197884 Free PMC article.
-
The combination of the opioid glycopeptide MMP-2200 and a NMDA receptor antagonist reduced l-DOPA-induced dyskinesia and MMP-2200 by itself reduced dopamine receptor 2-like agonist-induced dyskinesia.Neuropharmacology. 2018 Oct;141:260-271. doi: 10.1016/j.neuropharm.2018.09.005. Epub 2018 Sep 7. Neuropharmacology. 2018. PMID: 30201210 Free PMC article.
References
-
- Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, Jones H, Yamamura HI, Janders J, Davis TP, Porreca F, Hruby VJ, Polt R. Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J Med Chem. 2000;43:2586–90. - PubMed
-
- Breslin MB, Lindberg I, Benjannet S, Mathis JP, Lazure C, Seidah NG. Differential processing of proenkephalin by prohormone convertases 1(3) and 2 and furin. J Biol Chem. 1993;268:27084–93. - PubMed
-
- Calon F, Birdi S, Rajput AH, Hornykiewicz O, Bedard PJ, Di Paolo T. Increase of preproenkephalin mRNA levels in the putamen of Parkinson disease patients with levodopa-induced dyskinesias. J Neuropathol Exp Neurol. 2002;61:186–96. - PubMed
-
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

