IFN-λs
- PMID: 21840693
- PMCID: PMC3196341
- DOI: 10.1016/j.coi.2011.07.007
IFN-λs
Abstract
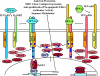
For decades, type I IFNs have been considered indispensable and unique antiviral mediators for the activation of rapid innate antiviral protection. However, the recent discovery of type III IFNs is challenging this paradigm. Since their identification in 2002/2003 by two independent groups, type III IFNs or IFN-λs, also known as IL-28/29, have been the subject of increased study with consequent recognition of their importance in virology and immunology. Initial reports suggested that IFN-λs functionally resemble type I IFNs. Although IFN-λs and classical type I IFNs (IFN-α/β) utilize distinct receptor complexes for signaling, both types of IFNs activate similar intracellular signaling pathways and biological activities, including the ability to induce antiviral state in cells, and both type I and type III IFNs are induced by viral infection. However, different antiviral potency, pattern of their induction and differential tissue expression of their corresponding receptor subunits suggest that the type I and type III IFN antiviral systems do not merely duplicate each other. Recent studies have started to reveal unique biological activities of IFN-λs in and beyond innate antiviral immunity.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat.Immunol. 2003;4:69–77. - PubMed
-
**This report, along with Ref. [2], describes identification and initial characterization of IFN-λs, their receptors and biological activities.
-
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat.Immunol. 2003;4:63–68. - PubMed
-
**This report, along with Ref. [1], describes identification and initial characterization of IFN-λs, their receptors and biological activities.
-
- Kotenko SV, Langer JA. Full house: 12 receptors for 27 cytokines. Int.Immunopharmacol. 2004;4:593–608. - PubMed
-
- Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. - PubMed
-
- Langer JA, Cutrone EC, Kotenko S. The Class II cytokine receptor (CRF2) family: overview and patterns of receptor-ligand interactions. Cytokine Growth Factor Rev. 2004;15:33–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

