Cross-talk between phospho-STAT3 and PLCγ1 plays a critical role in colorectal tumorigenesis
- PMID: 21840932
- PMCID: PMC3196678
- DOI: 10.1158/1541-7786.MCR-11-0147
Cross-talk between phospho-STAT3 and PLCγ1 plays a critical role in colorectal tumorigenesis
Abstract
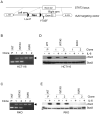
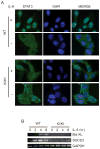
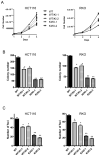
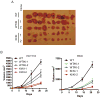
Hyperphosphorylation at the Y705 residue of signal transducer and activator of transcription 3 (STAT3) is implicated in tumorigenesis of leukemia and some solid tumors. However, its role in the development of colorectal cancer is not well defined. To rigorously test the impact of this phosphorylation on colorectal tumorigenesis, we engineered a STAT3 Y705F knock-in to interrupt STAT3 activity in HCT116 and RKO colorectal cancer cells. These STAT3 Y705F mutant cells fail to respond to cytokine stimulation and grow slower than parental cells. These mutant cells are also greatly diminished in their abilities to form colonies in culture, to exhibit anchorage-independent growth in soft agar, and to grow as xenografts in nude mice. These observations strongly support the premise that STAT3 Y705 phosphorylation is crucial in colorectal tumorigenesis. Although it is generally believed that STAT3 functions as a transcription factor, recent studies indicate that transcription-independent functions of STAT3 also play an important role in tumorigenesis. We show here that wild-type STAT3, but not STAT3 Y705F mutant protein, associates with phospholipase Cγ1 (PLCγ1). PLCγ1 is a central signal transducer of growth factor and cytokine signaling pathways that are involved in tumorigenesis. In STAT3 Y705F mutant colorectal cancer cells, PLCγ1 activity is reduced. Moreover, overexpression of a constitutively active form of PLCγ1 rescues the transformation defect of STAT3 Y705F mutant cells. In aggregate, our study identifies previously unknown cross-talk between STAT3 and the PLCγ signaling pathways that may play a critical role in colorectal tumorigenesis.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

