Lipoarabinomannan localization and abundance during growth of Mycobacterium smegmatis
- PMID: 21840972
- PMCID: PMC3187192
- DOI: 10.1128/JB.05299-11
Lipoarabinomannan localization and abundance during growth of Mycobacterium smegmatis
Abstract
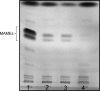
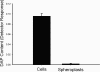
Lipoarabinomannan (LAM) is a structurally heterogeneous amphipathic lipoglycan present in Mycobacterium spp. and other actinomycetes, which constitutes a major component of the cell wall and exhibits a wide spectrum of immunomodulatory effects. Analysis of Mycobacterium smegmatis subcellular fractions and spheroplasts showed that LAM and lipomannan (LM) were primarily found in a cell wall-enriched subcellular fraction and correlated with the presence (or absence) of the mycolic acids in spheroplast preparations, suggesting that LAM and LM are primarily associated with the putative outer membrane of mycobacteria. During the course of these studies significant changes in the LAM/LM content of the cell wall were noted relative to the age of the culture. The LAM content of the M. smegmatis cell wall was dramatically reduced as the bacilli approached stationary phase, whereas LM, mycolic acid, and arabinogalactan content appeared to be unchanged. In addition, cell morphology and acid-fast staining characteristics showed variations with growth phase of the bacteria. In the logarithmic phase, the bacteria were found to be classic rod-shaped acid-fast bacilli, while in the stationary phase M. smegmatis lost the characteristic rod shape and developed a punctate acid-fast staining pattern with carbolfuchsin. The number of viable bacteria was independent of LAM content and phenotype. Taken together, the results presented here suggest that LAM is primarily localized with the mycolic acids in the cell wall and that the cellular concentration of LAM in M. smegmatis is selectively modulated with the growth phase.
Figures




References
-
- Alderwick L. J., Birch H. L., Mishra A., Eggeling L., Besra G. S. 2007. Structure, function and biosynthesis of the Mycobacterium tuberculosis cell wall: arabinogalactan and lipoarabinomannan assembly with a view to discovering new drug targets. Biochem. Soc. Trans. 35:1325–1328 - PubMed
-
- Alsteens D., et al. 2008. Organization of the mycobacterial cell wall: a nanoscale view. Eur. J. Physiol. 456:117–125 - PubMed
-
- Besra G. S. 1998. Preparation of cell wall fractions from mycobacteria. Methods Mol. Biol. 101:91–107 - PubMed
-
- Betts J. C., Lukey P. T., Robb L. C., McAdam R. A., Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717–731 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

