microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects
- PMID: 21841015
- PMCID: PMC3197368
- DOI: 10.1152/ajpheart.01080.2010
microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects
Abstract
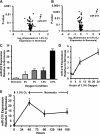
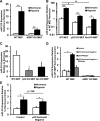
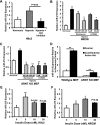
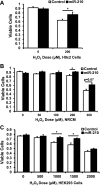
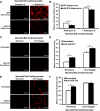
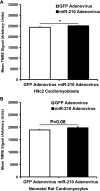
microRNA-210 (miR-210) is upregulated in hypoxia, but its function in cardiomyocytes and its regulation in response to hypoxia are not well characterized. The purpose of this study was to identify upstream regulators of miR-210, as well as to characterize miR-210's function in cardiomyocytes. We first showed miR-210 is upregulated through both hypoxia-inducible factor (HIF)-dependent and -independent pathways, since aryl hydrocarbon nuclear translocator (ARNT) knockout mouse embryonic fibroblasts (MEF), lacking intact HIF signaling, still displayed increased miR-210 levels in hypoxia. To determine the mechanism for HIF-independent regulation of miR-210, we focused on p53 and protein kinase B (Akt). Overexpression of p53 in wild-type MEFs induced miR-210, whereas p53 overexpression in ARNT knockout MEFs did not, suggesting p53 regulates miR-210 in a HIF-dependent mechanism. Akt inhibition reduced miR-210 induction by hypoxia, whereas Akt overexpression increased miR-210 levels in both wild-type and ARNT knockout MEFs, indicating Akt regulation of miR-210 is HIF-independent. We then studied the effects of miR-210 in cardiomyocytes. Overexpression of miR-210 reduced cell death in response to oxidative stress and reduced reactive oxygen species (ROS) production both at baseline and after treatment with antimycin A. Furthermore, downregulation of miR-210 increased ROS after hypoxia-reoxygenation. To determine a mechanism for the cytoprotective effects of miR-210, we focused on the predicted target, apoptosis-inducing factor, mitochondrion-associated 3 (AIFM3), known to induce cell death. Although miR-210 reduced AIFM3 levels, overexpression of AIFM3 in the presence of miR-210 overexpression did not reduce cellular viability either at baseline or after hydrogen peroxide treatment, suggesting AIFM3 does not mediate miR-210's cytoprotective effects. Furthermore, HIF-3α, a negative regulator of HIF signaling, is targeted by miR-210, but miR-210 does not modulate HIF activity. In conclusion, we demonstrate a novel role for p53 and Akt in regulating miR-210 and demonstrate that, in cardiomyocytes, miR-210 exerts cytoprotective effects, potentially by reducing mitochondrial ROS production.
Figures


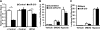
References
-
- Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet 5: 396–400, 2004 - PubMed
-
- Beitner-Johnson D, Rust RT, Hsieh TC, Millhorn DE. Hypoxia activates Akt and induces phosphorylation of GSK-3 in PC12 cells. Cell Signal 13: 23–27, 2001 - PubMed
-
- Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1: 409–414, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

