New insights into HIV assembly and trafficking
- PMID: 21841072
- PMCID: PMC3467973
- DOI: 10.1152/physiol.00051.2010
New insights into HIV assembly and trafficking
Abstract
Assembly and release of human immunodeficiency virus type 1 (HIV-1) particles is mediated by the viral Gag polyprotein precursor. Gag is synthesized in the cytosol and rapidly translocates to membrane to orchestrate particle production. The cell biology of HIV-1 Gag trafficking is currently one of the least understood aspects of HIV-1 replication. In this review, we highlight the current understanding of the cellular machinery involved in Gag trafficking and virus assembly.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures

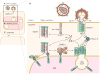
 ) matrix (MA) domain, capsid (CA), nucleocapsid (NC), p6 domains, and the spacer peptides SP1 and SP2 are indicated.
) matrix (MA) domain, capsid (CA), nucleocapsid (NC), p6 domains, and the spacer peptides SP1 and SP2 are indicated.


References
-
- Adamson CS, Freed EO. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv Pharmacol. 2007;55:347–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

