Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice
- PMID: 21841308
- PMCID: PMC3163966
- DOI: 10.1172/JCI57762
Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice
Abstract
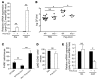
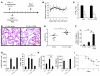
Pneumococcal infection of the respiratory tract is often secondary to recent influenza virus infection and accounts for much of the morbidity and mortality during seasonal and pandemic influenza. Here, we show that coinfection of the upper respiratory tract of mice with influenza virus and pneumococcus leads to synergistic stimulation of type I IFNs and that this impairs the recruitment of macrophages, which are required for pneumococcal clearance, due to decreased production of the chemokine CCL2. Type I IFN expression was induced by pneumococcal colonization alone. Colonization followed by influenza coinfection led to a synergistic type I IFN response, resulting in increased density of colonizing bacteria and susceptibility to invasive infection. This enhanced type I IFN response inhibited production of the chemokine CCL2, which promotes the recruitment of macrophages and bacterial clearance. Stimulation of CCL2 by macrophages upon pneumococcal infection alone required the pattern recognition receptor Nod2 and expression of the pore-forming toxin pneumolysin. Indeed, the increased colonization associated with concurrent influenza virus infection was not observed in mice lacking Nod2 or the type I IFN receptor, or in mice challenged with pneumococci lacking pneumolysin. We therefore propose that the synergistic stimulation of type I IFN production during concurrent influenza virus and pneumococcal infection leads to increased bacterial colonization and suggest that this may contribute to the higher rates of disease associated with coinfection in humans.
Figures

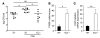

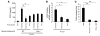
References
-
- Vu HT, et al. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. . Pediatr Infect Dis J. 2011;30(1):11–18. - PubMed
-
- Tong HH, Weiser JN, James MA, DeMaria TF. Effect of influenza A virus infection on nasopharyngeal colonization and otitis media induced by transparent or opaque phenotype variants of Streptococcus pneumoniae in the chinchilla model. . Infect Immun. 2001;69(1):602–606. doi: 10.1128/IAI.69.1.602-606.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

