Characterization of noninnocent metal complexes using solid-state NMR spectroscopy: o-dioxolene vanadium complexes
- PMID: 21842875
- PMCID: PMC3386638
- DOI: 10.1021/ic200046k
Characterization of noninnocent metal complexes using solid-state NMR spectroscopy: o-dioxolene vanadium complexes
Abstract
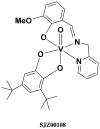
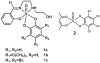
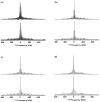
(51)V solid-state NMR (SSNMR) studies of a series of noninnocent vanadium(V) catechol complexes have been conducted to evaluate the possibility that (51)V NMR observables, quadrupolar and chemical shift anisotropies, and electronic structures of such compounds can be used to characterize these compounds. The vanadium(V) catechol complexes described in these studies have relatively small quadrupolar coupling constants, which cover a surprisingly small range from 3.4 to 4.2 MHz. On the other hand, isotropic (51)V NMR chemical shifts cover a wide range from -200 to 400 ppm in solution and from -219 to 530 ppm in the solid state. A linear correlation of (51)V NMR isotropic solution and solid-state chemical shifts of complexes containing noninnocent ligands is observed. These experimental results provide the information needed for the application of (51)V SSNMR spectroscopy in characterizing the electronic properties of a wide variety of vanadium-containing systems and, in particular, those containing noninnocent ligands and that have chemical shifts outside the populated range of -300 to -700 ppm. The studies presented in this report demonstrate that the small quadrupolar couplings covering a narrow range of values reflect the symmetric electronic charge distribution, which is also similar across these complexes. These quadrupolar interaction parameters alone are not sufficient to capture the rich electronic structure of these complexes. In contrast, the chemical shift anisotropy tensor elements accessible from (51)V SSNMR experiments are a highly sensitive probe of subtle differences in electronic distribution and orbital occupancy in these compounds. Quantum chemical (density functional theory) calculations of NMR parameters for [VO(hshed)(Cat)] yield a (51)V chemical shift anisotropy tensor in reasonable agreement with the experimental results, but surprisingly the calculated quadrupolar coupling constant is significantly greater than the experimental value. The studies demonstrate that substitution of the catechol ligand with electron-donating groups results in an increase in the HOMO-LUMO gap and can be directly followed by an upfield shift for the vanadium catechol complex. In contrast, substitution of the catechol ligand with electron-withdrawing groups results in a decrease in the HOMO-LUMO gap and can directly be followed by a downfield shift for the complex. The vanadium catechol complexes were used in this work because (51)V is a half-integer quadrupolar nucleus whose NMR observables are highly sensitive to the local environment. However, the results are general and could be extended to other redox-active complexes that exhibit coordination chemistry similar to that of the vanadium catechol complexes.
Figures




Similar articles
-
Effect of Ancillary Ligand on Electronic Structure as Probed by 51V Solid-State NMR Spectroscopy for Vanadium-o-Dioxolene Complexes.CrystEngComm. 2013 Nov 21;15(43):10.1039/C3CE41322E. doi: 10.1039/C3CE41322E. CrystEngComm. 2013. PMID: 24353476 Free PMC article.
-
Investigating the vanadium environments in hydroxylamido V(V) dipicolinate complexes using 51V NMR spectroscopy and density functional theory.Inorg Chem. 2007 Oct 29;46(22):9285-93. doi: 10.1021/ic7012667. Epub 2007 Sep 29. Inorg Chem. 2007. PMID: 17902653 Free PMC article.
-
(51)V solid-state NMR and density functional theory studies of eight-coordinate non-oxo vanadium complexes: oxidized amavadin.Dalton Trans. 2009 May 7;(17):3262-9. doi: 10.1039/b820383k. Epub 2009 Mar 13. Dalton Trans. 2009. PMID: 19421628 Free PMC article.
-
Advanced solid-state NMR spectroscopy and its applications in zeolite chemistry.Prog Nucl Magn Reson Spectrosc. 2024 Apr-Jun;140-141:1-41. doi: 10.1016/j.pnmrs.2023.11.001. Epub 2023 Nov 22. Prog Nucl Magn Reson Spectrosc. 2024. PMID: 38705634 Review.
-
Hydrolysis, Ligand Exchange, and Redox Properties of Vanadium Compounds: Implications of Solution Transformation on Biological, Therapeutic, and Environmental Applications.Chem Rev. 2025 Feb 12;125(3):1468-1603. doi: 10.1021/acs.chemrev.4c00475. Epub 2025 Jan 16. Chem Rev. 2025. PMID: 39818783 Review.
Cited by
-
Effect of positional isomerism and vanadium substitution on 51V magic angle spinning NMR Spectra Of Wells-Dawson polyoxotungstates.Solid State Nucl Magn Reson. 2017 Jul-Aug;84:28-33. doi: 10.1016/j.ssnmr.2016.12.004. Epub 2016 Dec 10. Solid State Nucl Magn Reson. 2017. PMID: 27998683 Free PMC article.
-
Quantum Mimicry With Inorganic Chemistry.Comments Mod Chem A Comments Inorg Chem. 2024;44(1):11-53. doi: 10.1080/02603594.2023.2173588. Epub 2023 Feb 13. Comments Mod Chem A Comments Inorg Chem. 2024. PMID: 38515928 Free PMC article.
-
Bioinspired design of redox-active ligands for multielectron catalysis: effects of positioning pyrazine reservoirs on cobalt for electro- and photocatalytic generation of hydrogen from water.Chem Sci. 2015 Aug 1;6(8):4954-4972. doi: 10.1039/c5sc01414j. Epub 2015 Jun 9. Chem Sci. 2015. PMID: 29142725 Free PMC article.
-
Effect of Ancillary Ligand on Electronic Structure as Probed by 51V Solid-State NMR Spectroscopy for Vanadium-o-Dioxolene Complexes.CrystEngComm. 2013 Nov 21;15(43):10.1039/C3CE41322E. doi: 10.1039/C3CE41322E. CrystEngComm. 2013. PMID: 24353476 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

