Polyamines are traps for reactive intermediates in furan metabolism
- PMID: 21842885
- PMCID: PMC3221807
- DOI: 10.1021/tx200273z
Polyamines are traps for reactive intermediates in furan metabolism
Abstract
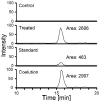
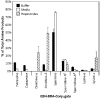
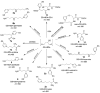
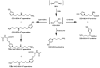
Furan is toxic and carcinogenic in rodents. Because of the large potential for human exposure, furan is classified as a possible human carcinogen. The detailed mechanism by which furan causes toxicity and cancer is not yet known. Since furan toxicity requires cytochrome P450-catalyzed oxidation of furan, we have characterized the urinary and hepatocyte metabolites of furan to gain insight into the chemical nature of the reactive intermediate. Previous studies in hepatocytes indicated that furan is oxidized to the reactive α,β-unsaturated dialdehyde, cis-2-butene-1,4-dial (BDA), which reacts with glutathione (GSH) to form 2-(S-glutathionyl)succinaldehyde (GSH-BDA). This intermediate forms pyrrole cross-links with cellular amines such as lysine and glutamine. In this article, we demonstrate that GSH-BDA also forms cross-links with ornithine, putrescine, and spermidine when furan is incubated with rat hepatocytes. The relative levels of these metabolites are not completely explained by hepatocellular levels of the amines or by their reactivity with GSH-BDA. Mercapturic acid derivatives of the spermidine cross-links were detected in the urine of furan-treated rats, which indicates that this metabolic pathway occurs in vivo. Their detection in furan-treated hepatocytes and in urine from furan-treated rats indicates that polyamines may play an important role in the toxicity of furan.
Figures




References
-
- National Toxicology Program. Toxicology and carcinogenesis studies of furan in F344/N rats and B6C3F1 mice vol NTP Technical Report No 402. US Department of Health and Human Services, Public Health Service, National Institutes of Health; Research Triangle Park, NC: 1993.
-
- International Agency for Research on Cancer. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. IARC; Lyon, France: 1995. Furan; p. 393.
-
- Perez LC, Yaylayan VA. Origin and mechanistic pathways of formation of the parent furan--a food toxicant. J Agric Food Chem. 2004;52:6830–6836. - PubMed
-
- National Toxicology Program. 11th Report on Carcinogens. US Department of Health and Human Services; Washington DC: 2005.
-
- Carfagna MA, Held SD, Kedderis GL. Furan-induced cytolethality in isolated rat hepatocytes: Correspondence with in vivo dosimetry. Toxicol Appl Pharmacol. 1993;123:265–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

