Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments
- PMID: 21842954
- PMCID: PMC5017532
- DOI: 10.1063/1.3608916
Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments
Abstract
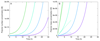
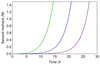
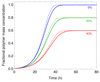
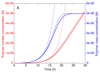
Self-assembly processes resulting in linear structures are often observed in molecular biology, and include the formation of functional filaments such as actin and tubulin, as well as generally dysfunctional ones such as amyloid aggregates. Although the basic kinetic equations describing these phenomena are well-established, it has proved to be challenging, due to their non-linear nature, to derive solutions to these equations except for special cases. The availability of general analytical solutions provides a route for determining the rates of molecular level processes from the analysis of macroscopic experimental measurements of the growth kinetics, in addition to the phenomenological parameters, such as lag times and maximal growth rates that are already obtainable from standard fitting procedures. We describe here an analytical approach based on fixed-point analysis, which provides self-consistent solutions for the growth of filamentous structures that can, in addition to elongation, undergo internal fracturing and monomer-dependent nucleation as mechanisms for generating new free ends acting as growth sites. Our results generalise the analytical expression for sigmoidal growth kinetics from the Oosawa theory for nucleated polymerisation to the case of fragmenting filaments. We determine the corresponding growth laws in closed form and derive from first principles a number of relationships which have been empirically established for the kinetics of the self-assembly of amyloid fibrils.
Figures








References
-
- Gibbs JW. On the equilibrium of heterogeneous substances. Trans Conn Acad Arts Sci. 1878;3:108, 343.
-
- Volmer M, Weber A. Keimbildung in übersättigten Gebilden. Z Phys Chem. 1926;119:277.
-
- Kaischew R, Stranski IN. The theory of the linear rate of crystallisation. Z phys Chem. 1934;A170:295.
-
- Stranski IN, Kaischew R. Crystal growth and crystal nucleation. Z Phys. 1935;36:393.
-
- Becker R, Döring W. Kinetische Behandlung der Keimbildung in übersättigten Dämpfen. Ann Phys. 1935;26:719. 5.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

