Lassa hemorrhagic fever in a late term pregnancy from northern Sierra Leone with a positive maternal outcome: case report
- PMID: 21843352
- PMCID: PMC3177908
- DOI: 10.1186/1743-422X-8-404
Lassa hemorrhagic fever in a late term pregnancy from northern Sierra Leone with a positive maternal outcome: case report
Erratum in
- Virol J. 2011;8:480. Zaitsev, Eleina M [added]
Abstract
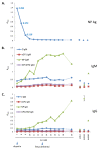
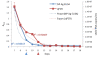
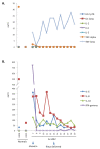
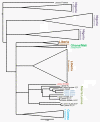
Lassa fever (LF) is a devastating viral disease prevalent in West Africa. Efforts to take on this public health crisis have been hindered by lack of infrastructure and rapid field deployable diagnosis in areas where the disease is prevalent. Recent capacity building at the Kenema Government Hospital Lassa Fever Ward (KGH LFW) in Sierra Leone has lead to a major turning point in the diagnosis, treatment and study of LF. Herein we present the first comprehensive rapid diagnosis and real time characterization of an acute hemorrhagic LF case at KGH LFW. This case report focuses on a third trimester pregnant Sierra Leonean woman from the historically non-endemic Northern district of Tonkolili who survived the illness despite fetal demise. Employed in this study were newly developed recombinant LASV Antigen Rapid Test cassettes and dipstick lateral flow immunoassays (LFI) that enabled the diagnosis of LF within twenty minutes of sample collection. Deregulation of overall homeostasis, significant hepatic and renal system involvement, and immunity profiles were extensively characterized during the course of hospitalization. Rapid diagnosis, prompt treatment with a full course of intravenous (IV) ribavirin, IV fluids management, and real time monitoring of clinical parameters resulted in a positive maternal outcome despite admission to the LFW seven days post onset of symptoms, fetal demise, and a natural still birth delivery. These studies solidify the growing rapid diagnostic, treatment, and surveillance capabilities at the KGH LF Laboratory, and the potential to significantly improve the current high mortality rate caused by LF. As a result of the growing capacity, we were also able to isolate Lassa virus (LASV) RNA from the patient and perform Sanger sequencing where we found significant genetic divergence from commonly circulating Sierra Leonean strains, showing potential for the discovery of a newly emerged LASV strain with expanded geographic distribution. Furthermore, recent emergence of LF cases in Northern Sierra Leone highlights the need for superior diagnostics to aid in the monitoring of LASV strain divergence with potentially increased geographic expansion.
Figures


References
-
- Buckley SM, Casals J. Lassa fever, a new virus disease of man from West Africa. Isolation and characterization of the virus. Am J Trop Med Hyg. 1970;19(4):680–691. - PubMed
-
- Birmingham K, Kenyon G. Lassa fever is unheralded problem in West Africa. Nat Med. 2001;7(8):878. - PubMed
-
- Fisher-Hoch SP, McCormick JB. Lassa fever vaccine: A review. Expert Rev Vaccines. 2004;3:103–111. - PubMed
-
- McCormick JB. Epidemiology and control of Lassa fever. Current Topics in Microbiol and Immunol. 1987;134:69–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

