Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus
- PMID: 21843872
- PMCID: PMC3157011
- DOI: 10.1016/j.chom.2011.07.004
Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus
Abstract
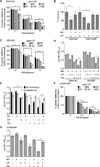
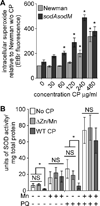
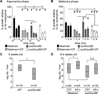
By sequestering manganese and zinc, the neutrophil protein calprotectin plays a crucial role in host defense against bacterial and fungal pathogens. However, the essential processes disrupted by calprotectin remain unknown. We report that calprotectin enhances the sensitivity of Staphylococcus aureus to superoxide through inhibition of manganese-dependent bacterial superoxide defenses, thereby increasing superoxide levels within the bacterial cell. Superoxide dismutase activity is required for full virulence in a systemic model of S. aureus infection, and disruption of staphylococcal superoxide defenses by calprotectin augments the antimicrobial activity of neutrophils promoting in vivo clearance. Calprotectin mutated in two transition metal binding sites and therefore defective in binding manganese and zinc does not inhibit microbial growth, unequivocally linking the antimicrobial properties of calprotectin to metal chelation. These results suggest that calprotectin contributes to host defense by rendering bacterial pathogens more sensitive to host immune effectors and reducing bacterial growth.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures

Comment in
-
Host response: test of mettle for neutrophils.Nat Rev Microbiol. 2011 Sep 16;9(10):700. doi: 10.1038/nrmicro2659. Nat Rev Microbiol. 2011. PMID: 21921931 No abstract available.
References
-
- Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5:3173–3178. - PubMed
-
- Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13:1205–1218. - PubMed
-
- Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. - PubMed
-
- Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994;55:253–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI069233/AI/NIAID NIH HHS/United States
- T32 CA009582/CA/NCI NIH HHS/United States
- T32 GM07347/GM/NIGMS NIH HHS/United States
- 1 R01GM62112/GM/NIGMS NIH HHS/United States
- R01AI069233/AI/NIAID NIH HHS/United States
- T32 HL094296/HL/NHLBI NIH HHS/United States
- R01 AI073843/AI/NIAID NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- R01 GM062112/GM/NIGMS NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- T32HL094296-02/HL/NHLBI NIH HHS/United States
- 5 T32 CA009582-23/CA/NCI NIH HHS/United States
- R01AI073843/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

