Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy
- PMID: 21844097
- PMCID: PMC3178293
- DOI: 10.2337/db10-1395
Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy
Abstract
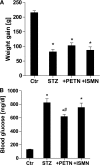
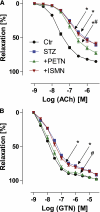
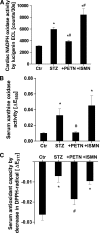
Objective: Diabetes is associated with vascular oxidative stress, activation of NADPH oxidase, and uncoupling of nitric oxide (NO) synthase (endothelial NO synthase [eNOS]). Pentaerithrityl tetranitrate (PETN) is an organic nitrate with potent antioxidant properties via induction of heme oxygenase-1 (HO-1). We tested whether treatment with PETN improves vascular dysfunction in the setting of experimental diabetes.
Research design and methods: After induction of hyperglycemia by streptozotocin (STZ) injection (60 mg/kg i.v.), PETN (15 mg/kg/day p.o.) or isosorbide-5-mononitrate (ISMN; 75 mg/kg/day p.o.) was fed to Wistar rats for 7 weeks. Oxidative stress was assessed by optical methods and oxidative protein modifications, vascular function was determined by isometric tension recordings, protein expression was measured by Western blotting, RNA expression was assessed by quantitative RT-PCR, and HO-1 promoter activity in stable transfected cells was determined by luciferase assays.
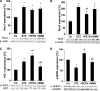
Results: PETN, but not ISMN, improved endothelial dysfunction. NADPH oxidase and serum xanthine oxidase activities were significantly reduced by PETN but not by ISMN. Both organic nitrates had minor effects on the expression of NADPH oxidase subunits, eNOS and dihydrofolate reductase (Western blotting). PETN, but not ISMN, normalized the expression of GTP cyclohydrolase-1, extracellular superoxide dismutase, and S-glutathionylation of eNOS, thereby preventing eNOS uncoupling. The expression of the antioxidant enzyme, HO-1, was increased by STZ treatment and further upregulated by PETN, but not ISMN, via activation of the transcription factor NRF2.
Conclusions: In contrast to ISMN, the organic nitrate, PETN, improves endothelial dysfunction in diabetes by preventing eNOS uncoupling and NADPH oxidase activation, thereby reducing oxidative stress. Thus, PETN therapy may be suited to treat patients with cardiovascular complications of diabetes.
Figures
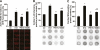
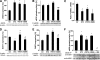

Similar articles
-
Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress.Antioxid Redox Signal. 2015 Oct 10;23(11):899-942. doi: 10.1089/ars.2015.6376. Epub 2015 Sep 24. Antioxid Redox Signal. 2015. PMID: 26261901 Free PMC article. Review.
-
Pentaerythritol tetranitrate improves angiotensin II-induced vascular dysfunction via induction of heme oxygenase-1.Hypertension. 2010 Apr;55(4):897-904. doi: 10.1161/HYPERTENSIONAHA.109.149542. Epub 2010 Feb 15. Hypertension. 2010. PMID: 20157049 Free PMC article.
-
Pentaerythrityl tetranitrate and nitroglycerin, but not isosorbide mononitrate, prevent endothelial dysfunction induced by ischemia and reperfusion.Arterioscler Thromb Vasc Biol. 2007 Sep;27(9):1955-9. doi: 10.1161/ATVBAHA.107.149278. Epub 2007 Jul 19. Arterioscler Thromb Vasc Biol. 2007. PMID: 17641250 Clinical Trial.
-
Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression.Eur Heart J. 2013 Nov;34(41):3206-16. doi: 10.1093/eurheartj/ehs100. Epub 2012 May 3. Eur Heart J. 2013. PMID: 22555214
-
Non-hemodynamic effects of organic nitrates and the distinctive characteristics of pentaerithrityl tetranitrate.Am J Cardiovasc Drugs. 2009;9(1):7-15. doi: 10.1007/BF03256591. Am J Cardiovasc Drugs. 2009. PMID: 19178128 Review.
Cited by
-
Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair.Redox Biol. 2015 Aug;5:275-289. doi: 10.1016/j.redox.2015.05.008. Epub 2015 Jun 3. Redox Biol. 2015. PMID: 26079210 Free PMC article. Review.
-
Regulation of Vascular Function and Inflammation via Cross Talk of Reactive Oxygen and Nitrogen Species from Mitochondria or NADPH Oxidase-Implications for Diabetes Progression.Int J Mol Sci. 2020 May 12;21(10):3405. doi: 10.3390/ijms21103405. Int J Mol Sci. 2020. PMID: 32408480 Free PMC article. Review.
-
The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity.PLoS One. 2014 Nov 17;9(11):e112394. doi: 10.1371/journal.pone.0112394. eCollection 2014. PLoS One. 2014. PMID: 25402275 Free PMC article.
-
Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress.Antioxid Redox Signal. 2014 Jan 10;20(2):308-24. doi: 10.1089/ars.2012.4609. Epub 2012 Jul 13. Antioxid Redox Signal. 2014. PMID: 22657349 Free PMC article. Review.
-
Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress.Antioxid Redox Signal. 2015 Oct 10;23(11):899-942. doi: 10.1089/ars.2015.6376. Epub 2015 Sep 24. Antioxid Redox Signal. 2015. PMID: 26261901 Free PMC article. Review.
References
-
- Nathan DM, Cleary PA, Backlund JY, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 - PMC - PubMed
-
- Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med 2006;40:183–192 - PubMed
-
- Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 2001;88:E14–E22 - PubMed
-
- Wenzel P, Schulz E, Oelze M, et al. AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic Biol Med 2008;45:619–626 - PubMed

