Proto-oncogene PBF/PTTG1IP regulates thyroid cell growth and represses radioiodide treatment
- PMID: 21844185
- PMCID: PMC3184940
- DOI: 10.1158/0008-5472.CAN-11-0720
Proto-oncogene PBF/PTTG1IP regulates thyroid cell growth and represses radioiodide treatment
Abstract
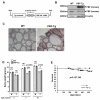
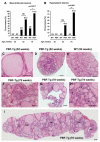
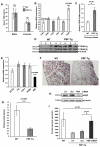
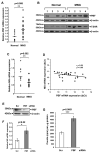
Pituitary tumor transforming gene (PTTG)-binding factor (PBF or PTTG1IP) is a little characterized proto-oncogene that has been implicated in the etiology of breast and thyroid tumors. In this study, we created a murine transgenic model to target PBF expression to the thyroid gland (PBF-Tg mice) and found that these mice exhibited normal thyroid function, but a striking enlargement of the thyroid gland associated with hyperplastic and macrofollicular lesions. Expression of the sodium iodide symporter (NIS), a gene essential to the radioiodine ablation of thyroid hyperplasia, neoplasia, and metastasis, was also potently inhibited in PBF-Tg mice. Critically, iodide uptake was repressed in primary thyroid cultures from PBF-Tg mice, which could be rescued by PBF depletion. PBF-Tg thyroids exhibited upregulation of Akt and the TSH receptor (TSHR), each known regulators of thyrocyte proliferation, along with upregulation of the downstream proliferative marker cyclin D1. We extended and confirmed findings from the mouse model by examining PBF expression in human multinodular goiters (MNG), a hyperproliferative thyroid disorder, where PBF and TSHR was strongly upregulated relative to normal thyroid tissue. Furthermore, we showed that depleting PBF in human primary thyrocytes was sufficient to increase radioiodine uptake. Together, our findings indicate that overexpression of PBF causes thyroid cell proliferation, macrofollicular lesions, and hyperplasia, as well as repression of the critical therapeutic route for radioiodide uptake.
Figures


References
-
- Boelaert K, Tannahill LA, Bulmer JN, Kachilele S, Chan SY, Kim D, et al. A potential role for PTTG/securin in the developing human fetal brain. Faseb J. 2003;17:1631–9. - PubMed
-
- Boelaert K, Smith VE, Stratford AL, Kogai T, Tannahill LA, Watkinson JC, et al. PTTG and PBF repress the human sodium iodide symporter. Oncogene. 2007;26:4344–56. - PubMed
-
- Chien W, Pei L. A novel binding factor facilitates nuclear translocation and transcriptional activation function of the pituitary tumor-transforming gene product. J Biol Chem. 2000;275:19422–7. - PubMed
-
- Mo Z, Zu X, Xie Z, Li W, Ning H, Jiang Y, et al. Antitumor effect of F-PBF(beta-TrCP)-induced targeted PTTG1 degradation in HeLa cells. J Biotechnol. 2009;139:6–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

