c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice
- PMID: 21844199
- PMCID: PMC3186406
- DOI: 10.1074/jbc.M111.276089
c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice
Abstract
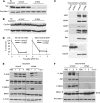
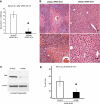
Sustained JNK activation plays a critical role in hepatotoxicity by acetaminophen or GalN/TNF-α. To address the importance of JNK translocation to mitochondria that accompanies sustained activation in these models, we assessed the importance of the expression of a potential initial target of JNK in the outer membrane of mitochondria, namely Sab (SH3 domain-binding protein that preferentially associates with Btk), also known as Sh3bp5 (SH3 domain-binding protein 5). Silencing the expression of Sab in the liver using adenoviral shRNA inhibited sustained JNK activation and mitochondrial targeting of JNK and the upstream MKK4 (MAPK kinase 4), accompanied by striking protection against liver injury in vivo and in cultured hepatocytes in both toxicity models. We conclude that mitochondrial Sab may serve as a platform for the MAPK pathway enzymes and that the interaction of stress-activated JNK with Sab is required for sustained JNK activation and toxicity.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

