Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis
- PMID: 21844207
- PMCID: PMC3160584
- DOI: 10.1083/jcb.201011119
Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis
Abstract
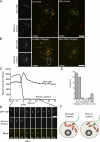
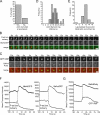
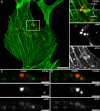
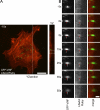
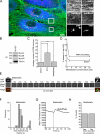
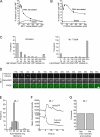
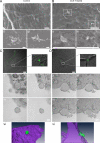
The study of actin in regulated exocytosis has a long history with many different results in numerous systems. A major limitation on identifying precise mechanisms has been the paucity of experimental systems in which actin function has been directly assessed alongside granule content release at distinct steps of exocytosis of a single secretory organelle with sufficient spatiotemporal resolution. Using dual-color confocal microscopy and correlative electron microscopy in human endothelial cells, we visually distinguished two sequential steps of secretagogue-stimulated exocytosis: fusion of individual secretory granules (Weibel-Palade bodies [WPBs]) and subsequent expulsion of von Willebrand factor (VWF) content. Based on our observations, we conclude that for fusion, WPBs are released from cellular sites of actin anchorage. However, once fused, a dynamic ring of actin filaments and myosin II forms around the granule, and actomyosin II contractility squeezes VWF content out into the extracellular environment. This study therefore demonstrates how discrete actin cytoskeleton functions within a single cellular system explain actin filament-based prevention and promotion of specific exocytic steps during regulated secretion.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

