Reducing plasma membrane sphingomyelin increases insulin sensitivity
- PMID: 21844222
- PMCID: PMC3187286
- DOI: 10.1128/MCB.05893-11
Reducing plasma membrane sphingomyelin increases insulin sensitivity
Abstract
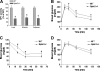
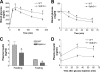
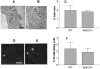
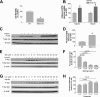
It has been shown that inhibition of de novo sphingolipid synthesis increases insulin sensitivity. For further exploration of the mechanism involved, we utilized two models: heterozygous serine palmitoyltransferase (SPT) subunit 2 (Sptlc2) gene knockout mice and sphingomyelin synthase 2 (Sms2) gene knockout mice. SPT is the key enzyme in sphingolipid biosynthesis, and Sptlc2 is one of its subunits. Homozygous Sptlc2-deficient mice are embryonic lethal. However, heterozygous Sptlc2-deficient mice that were viable and without major developmental defects demonstrated decreased ceramide and sphingomyelin levels in the cell plasma membranes, as well as heightened sensitivity to insulin. Moreover, these mutant mice were protected from high-fat diet-induced obesity and insulin resistance. SMS is the last enzyme for sphingomyelin biosynthesis, and SMS2 is one of its isoforms. Sms2 deficiency increased cell membrane ceramide but decreased SM levels. Sms2 deficiency also increased insulin sensitivity and ameliorated high-fat diet-induced obesity. We have concluded that Sptlc2 heterozygous deficiency- or Sms2 deficiency-mediated reduction of SM in the plasma membranes leads to an improvement in tissue and whole-body insulin sensitivity.
Copyright © 2011, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Adams J. M., II, et al. 2004. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 - PubMed
-
- de Mello V. D., et al. 2009. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia 52:2612–2615 - PubMed
-
- Drab M., et al. 2001. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293:2449–2452 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
