Evaluation of the antiviral response to zanamivir administered intravenously for treatment of critically ill patients with pandemic influenza A (H1N1) infection
- PMID: 21844304
- PMCID: PMC3156108
- DOI: 10.1093/infdis/jir397
Evaluation of the antiviral response to zanamivir administered intravenously for treatment of critically ill patients with pandemic influenza A (H1N1) infection
Abstract
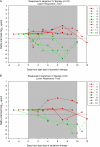
A retrospective nationwide study on the use of intravenous (IV) zanamivir in patients receiving intensive care who were pretreated with oseltamivir in the Netherlands was performed. In 6 of 13 patients with a sustained reduction of the viral load, the median time to start IV zanamivir was 9 days (range, 4-11 days) compared with 14 days (range, 6-21 days) in 7 patients without viral load reduction (P = .052). Viral load response did not influence mortality. We conclude that IV zanamivir as late add-on therapy has limited effectiveness. The effect of an immediate start with IV zanamivir monotherapy or in combination with other drugs need to be evaluated.
Figures
Comment in
-
Reply to Chan-Tack et al.J Infect Dis. 2013 Jan 1;207(1):198-9. doi: 10.1093/infdis/jis638. Epub 2012 Oct 22. J Infect Dis. 2013. PMID: 23089588 No abstract available.
-
Clinical experience with intravenous zanamivir under an emergency investigational new drug program in the United States.J Infect Dis. 2013 Jan 1;207(1):196-8. doi: 10.1093/infdis/jis637. Epub 2012 Oct 22. J Infect Dis. 2013. PMID: 23089591 Free PMC article. No abstract available.
References
-
- World Health Organization. Pandemic (H1N1) 2009. Geneva: WHO; 2010. http://www.who.int/csr/disease/swineflu/en/index.html. Accessed 25 October 2010.
-
- Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. - PubMed
-
- Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–7. - PubMed
-
- Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

