Efficacy of human-simulated exposures of tomopenem (formerly CS-023) in a murine model of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus infection
- PMID: 21844314
- PMCID: PMC3195026
- DOI: 10.1128/AAC.00068-11
Efficacy of human-simulated exposures of tomopenem (formerly CS-023) in a murine model of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus infection
Abstract
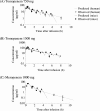
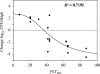
Tomopenem (formerly CS-023) is a novel carbapenem with improved activity against diverse hospital pathogens, including Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA), and has a half-life about twice longer than the half-lives of other carbapenems such as imipenem and meropenem. Our objective in this study was to estimate the efficacy of tomopenem in humans by human-simulated exposures in a neutropenic murine thigh infection model against 9 clinical isolates of P. aeruginosa with MICs of 4 to 32 μg/ml and 9 clinical isolates of MRSA with MICs of 4 to 16 μg/ml. Human-simulated dosing regimens in neutropenic mice were designed to approximate the cumulative percentage of a 24-h period that the free drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (f%T(MIC)) observed with tomopenem at 750 and 1,500 mg given as a 0.5-h infusion three times a day (TID) in humans. As reported previously, there was no difference between the target values of P. aeruginosa and MRSA required for efficacy (K. Sugihara et al., Antimicrob. Agents Chemother. 54:5298-5302, 2010). Tomopenem at 750 mg showed bactericidal or bacteriostatic effects against 10 of 11 strains of P. aeruginosa and MRSA with MICs of ≤ 8 μg/ml (f%T(MIC) ≥ 41), and tomopenem at 1,500 mg showed bactericidal effects against 16 of 17 strains of P. aeruginosa and MRSA with MICs of ≤ 16 μg/ml (f%T(MIC) ≥ 43). Meropenem at 1,000 mg TID was tested for comparison purposes and showed bactericidal or bacteriostatic effects against 3 of 4 strains of P. aeruginosa with MICs of ≤ 4 μg/ml (f%T(MIC) ≥ 33). From these results, tomopenem is expected to be effective with an f%T(MIC) of over 40 against P. aeruginosa and MRSA strains with MICs of ≤ 8 μg/ml at doses of 750 mg TID and strains with MICs of ≤ 16 μg/ml at doses of 1,500 mg TID.
Figures


Similar articles
-
In vivo pharmacodynamic activity of tomopenem (formerly CS-023) against Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus in a murine thigh infection model.Antimicrob Agents Chemother. 2010 Dec;54(12):5298-302. doi: 10.1128/AAC.00267-10. Epub 2010 Oct 4. Antimicrob Agents Chemother. 2010. PMID: 20921311 Free PMC article.
-
In vivo efficacy and pharmacokinetics of tomopenem (CS-023), a novel carbapenem, against Pseudomonas aeruginosa in a murine chronic respiratory tract infection model.J Antimicrob Chemother. 2008 Dec;62(6):1326-31. doi: 10.1093/jac/dkn411. Epub 2008 Oct 3. J Antimicrob Chemother. 2008. PMID: 18835805
-
Pharmacodynamics of the antibacterial effect and emergence of resistance to tomopenem, formerly RO4908463/CS-023, in an in vitro pharmacokinetic model of Staphylococcus aureus infection.Antimicrob Agents Chemother. 2008 Apr;52(4):1401-6. doi: 10.1128/AAC.01153-07. Epub 2008 Jan 28. Antimicrob Agents Chemother. 2008. PMID: 18227179 Free PMC article.
-
Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa.Semin Respir Crit Care Med. 2011 Apr;32(2):228-35. doi: 10.1055/s-0031-1275535. Epub 2011 Apr 19. Semin Respir Crit Care Med. 2011. PMID: 21506059 Review.
-
Delafloxacin: a novel fluoroquinolone with activity against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa.Expert Rev Anti Infect Ther. 2018 Jul;16(7):523-530. doi: 10.1080/14787210.2018.1489721. Epub 2018 Jun 26. Expert Rev Anti Infect Ther. 2018. PMID: 29911455 Review.
Cited by
-
Emerging Treatment Options for Infections by Multidrug-Resistant Gram-Positive Microorganisms.Microorganisms. 2020 Jan 30;8(2):191. doi: 10.3390/microorganisms8020191. Microorganisms. 2020. PMID: 32019171 Free PMC article. Review.
References
-
- Boucher H. W., et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 - PubMed
-
- Clinical Laboratory Standards Institute 2006. M7-A7. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA
-
- DeRyke C. A., Nicolau D. P. 2007. Is all free time above the minimum inhibitory concentration the same: implications for β-lactam in vivo modeling. Int. J. Antimicrob. Agents 29:341–343 - PubMed
-
- DeRyke C. A., Maglio D., Nicolau D. P. 2005. Defining the need for new antimicrobials: clinical and economic implications of resistance in the hospitalized patient. Expert Opin. Pharmacother. 6:873–889 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

