Parallel adaptive feedback enhances reliability of the Ca2+ signaling system
- PMID: 21844332
- PMCID: PMC3167526
- DOI: 10.1073/pnas.1018266108
Parallel adaptive feedback enhances reliability of the Ca2+ signaling system
Abstract
Despite large cell-to-cell variations in the concentrations of individual signaling proteins, cells transmit signals correctly. This phenomenon raises the question of what signaling systems do to prevent a predicted high failure rate. Here we combine quantitative modeling, RNA interference, and targeted selective reaction monitoring (SRM) mass spectrometry, and we show for the ubiquitous and fundamental calcium signaling system that cells monitor cytosolic and endoplasmic reticulum (ER) Ca(2+) levels and adjust in parallel the concentrations of the store-operated Ca(2+) influx mediator stromal interaction molecule (STIM), the plasma membrane Ca(2+) pump plasma membrane Ca-ATPase (PMCA), and the ER Ca(2+) pump sarco/ER Ca(2+)-ATPase (SERCA). Model calculations show that this combined parallel regulation in protein expression levels effectively stabilizes basal cytosolic and ER Ca(2+) levels and preserves receptor signaling. Our results demonstrate that, rather than directly controlling the relative level of signaling proteins in a forward regulation strategy, cells prevent transmission failure by sensing the state of the signaling pathway and using multiple parallel adaptive feedbacks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
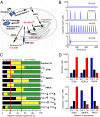
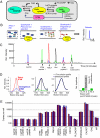


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

