Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts
- PMID: 21844347
- PMCID: PMC3167534
- DOI: 10.1073/pnas.1110412108
Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts
Abstract
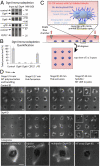
Female meiotic spindles in many organisms form in the absence of centrosomes, the organelle typically associated with microtubule (MT) nucleation. Previous studies have proposed that these meiotic spindles arise from RanGTP-mediated MT nucleation in the vicinity of chromatin; however, whether this process is sufficient for spindle formation is unknown. Here, we investigated whether a recently proposed spindle-based MT nucleation pathway that involves augmin, an 8-subunit protein complex, also contributes to spindle morphogenesis. We used an assay system in which hundreds of meiotic spindles can be observed forming around chromatin-coated beads after introduction of Xenopus egg extracts. Spindles forming in augmin-depleted extracts showed reduced rates of MT formation and were predominantly multipolar, revealing a function of augmin in stabilizing the bipolar shape of the acentrosomal meiotic spindle. Our studies also have uncovered an apparent augmin-independent MT nucleation process from acentrosomal poles, which becomes increasingly active over time and appears to partially rescue the spindle defects that arise from augmin depletion. Our studies reveal that spatially and temporally distinct MT generation pathways from chromatin, spindle MTs, and acentrosomal poles all contribute to robust bipolar spindle formation in meiotic extracts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wiese C, Zheng YX. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci. 2006;119:4143–4153. - PubMed
-
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. - PubMed
-
- Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol. 2000;10:59–67. - PubMed
-
- Megraw TL, Kao LR, Kaufman TC. Zygotic development without functional mitotic centrosomes. Curr Biol. 2001;11:116–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

