Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus
- PMID: 21844353
- PMCID: PMC3167523
- DOI: 10.1073/pnas.1110133108
Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus
Abstract
RIG-I is a cytosolic pathogen recognition receptor that engages viral RNA in infected cells to trigger innate immune defenses through its adaptor protein MAVS. MAVS resides on mitochondria and peroxisomes, but how its signaling is coordinated among these organelles has not been defined. Here we show that a major site of MAVS signaling is the mitochondrial-associated membrane (MAM), a distinct membrane compartment that links the endoplasmic reticulum to mitochondria. During RNA virus infection, RIG-I is recruited to the MAM to bind MAVS. Dynamic MAM tethering to mitochondria and peroxisomes then coordinates MAVS localization to form a signaling synapse between membranes. Importantly, the hepatitis C virus NS3/4A protease, which cleaves MAVS to support persistent infection, targets this synapse for MAVS proteolysis from the MAM, but not from mitochondria, to ablate RIG-I signaling of immune defenses. Thus, the MAM mediates an intracellular immune synapse that directs antiviral innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

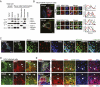

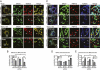
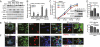
References
-
- Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. - PubMed
-
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell. 2005;122:669–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

