Innate C-H trifluoromethylation of heterocycles
- PMID: 21844378
- PMCID: PMC3167544
- DOI: 10.1073/pnas.1109059108
Innate C-H trifluoromethylation of heterocycles
Abstract
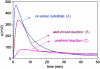
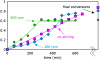
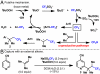
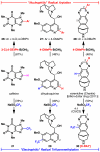
Direct methods for the trifluoromethylation of heteroaromatic systems are in extremely high demand in nearly every sector of chemical industry. Here we report the discovery of a general procedure using a benchtop stable trifluoromethyl radical source that functions broadly on a variety of electron deficient and rich heteroaromatic systems and demonstrates high functional group tolerance. This C-H trifluoromethylation protocol is operationally simple (avoids gaseous CF(3)I), scalable, proceeds at ambient temperature, can be used directly on unprotected molecules, and is demonstrated to proceed at the innately reactive positions of the substrate. The unique and orthogonal reactivity of the trifluoromethyl radical relative to aryl radicals has also been investigated on both a complex natural product and a pharmaceutical agent. Finally, preliminary data suggest that the regioselectivity of C-H trifluoromethylation can be fine-tuned simply by judicious solvent choice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



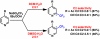
References
-
- Smart BE. Introduction: Fluorine chemistry. Chem Rev. 1996;96:1555–1556. - PubMed
-
- Filler R, Kobayashi Y, Yagupolskii LM, editors. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications. Amsterdam: Elsevier; 1993.
-
- Welch JT. Advances in the preparation of biologically-active organofluorine compounds. Tetrahedron. 1987;43:3123–3197.
-
- Ma JA, Cahard D. Strategies for nucleophilic, electrophilic, and radical trifluoromethylations. J Fluorine Chem. 2007;128:975–996.
-
- Meanwell NA. Synopsis of some recent tactical application of bioisosteres in drug design. J Med Chem. 2011;54:2529–2591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

