Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation
- PMID: 21844382
- PMCID: PMC3173876
- DOI: 10.4049/jimmunol.1100378
Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation
Abstract
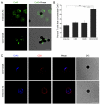
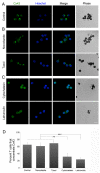
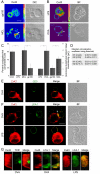
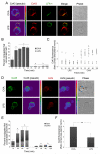
Gap junction (GJ) mediates intercellular communication through linked hemichannels from each of two adjacent cells. Using human and mouse models, we show that connexin 43 (Cx43), the main GJ protein in the immune system, was recruited to the immunological synapse during T cell priming as both GJs and stand-alone hemichannels. Cx43 accumulation at the synapse was Ag specific and time dependent, and required an intact actin cytoskeleton. Fluorescence recovery after photobleaching and Cx43-specific inhibitors were used to prove that intercellular communication between T cells and dendritic cells is bidirectional and specifically mediated by Cx43. Moreover, this intercellular cross talk contributed to T cell activation as silencing of Cx43 with an antisense or inhibition of GJ docking impaired intracellular Ca(2+) responses and cytokine release by T cells. These findings identify Cx43 as an important functional component of the immunological synapse and reveal a crucial role for GJs and hemichannels as coordinators of the dendritic cell-T cell signaling machinery that regulates T cell activation.
Figures

References
-
- Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. - PubMed
-
- Hedrick SM, Nielsen EA, Kavaler J, Cohen DI, Davis MM. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984;308:153–158. - PubMed
-
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. - PubMed
-
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. - PubMed
-
- Trautmann A, Valitutti S. The diversity of immunological synapses. Curr Opin Immunol. 2003;15:249–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

