Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide
- PMID: 21844879
- PMCID: PMC3374073
- DOI: 10.1038/ijo.2011.158
Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide
Erratum in
- Int J Obes (Lond). 2012 Jun;36(6):890
- Int J Obes (Lond). 2013 Feb;37(2):322
Abstract
Objective: Having demonstrated short-term weight loss with liraglutide in this group of obese adults, we now evaluate safety/tolerability (primary outcome) and long-term efficacy for sustaining weight loss (secondary outcome) over 2 years.
Design: A randomized, double-blind, placebo-controlled 20-week study with 2-year extension (sponsor unblinded at 20 weeks, participants/investigators at 1 year) in 19 European clinical research centers.
Subjects: A total of 564 adults (n=90-98 per group; body mass index 30-40 kg m(-2)) enrolled, 398 entered the extension and 268 completed the 2-year trial. Participants received diet (500 kcal deficit per day) and exercise counseling during 2-week run-in, before being randomly assigned (with a telephone or web-based system) to once-daily subcutaneous liraglutide (1.2, 1.8, 2.4 or 3.0 mg, n=90-95), placebo (n=98) or open-label orlistat (120 mg × 3, n=95). After 1 year, liraglutide/placebo recipients switched to liraglutide 2.4 mg, then 3.0 mg (based on 20-week and 1-year results, respectively). The trial ran from January 2007-April 2009 and is registered with Clinicaltrials.gov, number NCT00480909.
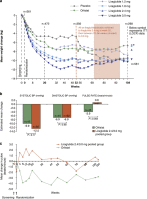
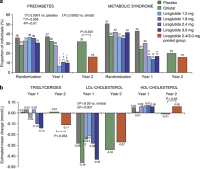
Results: From randomization to year 1, liraglutide 3.0 mg recipients lost 5.8 kg (95% confidence interval 3.7-8.0) more weight than those on placebo and 3.8 kg (1.6-6.0) more than those on orlistat (P0.0001; intention-to-treat, last-observation-carried-forward). At year 2, participants on liraglutide 2.4/3.0 mg for the full 2 years (pooled group, n=184) lost 3.0 kg (1.3-4.7) more weight than those on orlistat (n=95; P<0.001). Completers on liraglutide 2.4/3.0 mg (n=92) maintained a 2-year weight loss of 7.8 kg from screening. With liraglutide 3.0 mg, 20-week body fat decreased by 15.4% and lean tissue by 2.0%. The most frequent drug-related side effects were mild to moderate, transient nausea and vomiting. With liraglutide 2.4/3.0 mg, the 2-year prevalence of prediabetes and metabolic syndrome decreased by 52 and 59%, with improvements in blood pressure and lipids.
Conclusion: Liraglutide is well tolerated, sustains weight loss over 2 years and improves cardiovascular risk factors.
Figures

References
-
- Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. - PubMed
-
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. - PubMed
-
- National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes Res. 1998;6 (Suppl 2:51S–209S. - PubMed
-
- Mertens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8:270–278. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

