Transduction of the Hedgehog signal through the dimerization of Fused and the nuclear translocation of Cubitus interruptus
- PMID: 21844892
- PMCID: PMC3193457
- DOI: 10.1038/cr.2011.136
Transduction of the Hedgehog signal through the dimerization of Fused and the nuclear translocation of Cubitus interruptus
Abstract
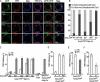
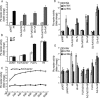
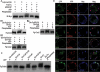
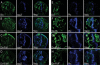
The Hedgehog (Hh) family of secreted proteins is essential for development in both vertebrates and invertebrates. As one of main morphogens during metazoan development, the graded Hh signal is transduced across the plasma membrane by Smoothened (Smo) through the differential phosphorylation of its cytoplasmic tail, leading to pathway activation and the differential expression of target genes. However, how Smo transduces the graded Hh signal via the Costal2 (Cos2)/Fused (Fu) complex remains poorly understood. Here we present a model of the cell response to a Hh gradient by translating Smo phosphorylation information to Fu dimerization and Cubitus interruptus (Ci) nuclear localization information. Our findings suggest that the phosphorylated C-terminus of Smo recruits the Cos2/Fu complex to the membrane through the interaction between Smo and Cos2, which further induces Fu dimerization. Dimerized Fu is phosphorylated and transduces the Hh signal by phosphorylating Cos2 and Suppressor of Fu (Su(fu)). We further show that this process promotes the dissociation of the full-length Ci (Ci155) and Cos2 or Su(fu), and results in the translocation of Ci155 into the nucleus, activating the expression of target genes.
Figures



References
-
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. - PubMed
-
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. - PubMed
-
- Pospisilik JA, Schramek D, Schnidar H, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. - PubMed
-
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

