Pulsed ultraviolet light reduces immunoglobulin E binding to Atlantic white shrimp (Litopenaeus setiferus) extract
- PMID: 21845146
- PMCID: PMC3155317
- DOI: 10.3390/ijerph8072569
Pulsed ultraviolet light reduces immunoglobulin E binding to Atlantic white shrimp (Litopenaeus setiferus) extract
Abstract
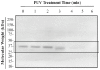
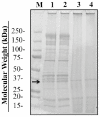
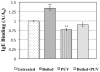
Pulsed ultraviolet light (PUV), a novel food processing and preservation technology, has been shown to reduce allergen levels in peanut and soybean samples. In this study, the efficacy of using PUV to reduce the reactivity of the major shrimp allergen, tropomyosin (36-kDa), and to attenuate immunoglobulin E (IgE) binding to shrimp extract was examined. Atlantic white shrimp (Litopenaeus setiferus) extract was treated with PUV (3 pulses/s, 10 cm from light source) for 4 min. Tropomyosin was compared in the untreated, boiled, PUV-treated and [boiled+PUV]-treated samples, and changes in the tropomyosin levels were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). IgE binding of the treated extract was analyzed via immunoblot and enzyme-linked immunosorbent assay (ELISA) using pooled human plasma containing IgE antibodies against shrimp allergens. Results showed that levels of tropomyosin and IgE binding were reduced following PUV treatment. However, boiling increased IgE binding, while PUV treatment could offset the increased allergen reactivity caused by boiling. In conclusion, PUV treatment reduced the reactivity of the major shrimp allergen, tropomyosin, and decreased the IgE binding capacity of the shrimp extract.
Keywords: IgE antibodies; PUV; allergen; allergy; pulsed ultraviolet light; shrimp; tropomyosin.
Figures



Similar articles
-
In vitro gastric and intestinal digestions of pulsed light-treated shrimp extracts.Appl Biochem Biotechnol. 2012 Mar;166(6):1409-22. doi: 10.1007/s12010-011-9534-2. Epub 2012 Jan 26. Appl Biochem Biotechnol. 2012. PMID: 22278049
-
Effects of boiling on the IgE-binding properties of tropomyosin of shrimp (Litopenaeus vannamei).J Food Sci. 2010 Jan-Feb;75(1):T1-5. doi: 10.1111/j.1750-3841.2009.01391.x. J Food Sci. 2010. PMID: 20492208
-
Molecular Diagnosis of Shrimp Allergy: Efficiency of Several Allergens to Predict Clinical Reactivity.J Allergy Clin Immunol Pract. 2015 Jul-Aug;3(4):521-9.e10. doi: 10.1016/j.jaip.2015.02.001. Epub 2015 Mar 11. J Allergy Clin Immunol Pract. 2015. PMID: 25769902 Clinical Trial.
-
Seafood allergy and allergens: a review.Mar Biotechnol (NY). 2003 Jul-Aug;5(4):339-48. doi: 10.1007/s10126-002-0082-1. Mar Biotechnol (NY). 2003. PMID: 14719162 Review.
-
Shellfish allergens: tropomyosin and beyond.Allergy. 2017 Jun;72(6):842-848. doi: 10.1111/all.13115. Epub 2017 Jan 19. Allergy. 2017. PMID: 28027402 Review.
Cited by
-
Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes in biofilms by pulsed ultraviolet light.BMC Res Notes. 2015 Jun 10;8:235. doi: 10.1186/s13104-015-1206-9. BMC Res Notes. 2015. PMID: 26054759 Free PMC article.
-
Effect of pulsed light treatment on enzymes and protein allergens associated with their structural changes: a review.J Food Sci Technol. 2021 Aug;58(8):2853-2862. doi: 10.1007/s13197-020-04882-9. Epub 2020 Nov 18. J Food Sci Technol. 2021. PMID: 34294948 Free PMC article. Review.
-
Research Progress on Shrimp Allergens and Allergenicity Reduction Methods.Foods. 2025 Mar 6;14(5):895. doi: 10.3390/foods14050895. Foods. 2025. PMID: 40077598 Free PMC article. Review.
-
Comprehensive Analysis of the Structure and Allergenicity Changes of Seafood Allergens Induced by Non-Thermal Processing: A Review.Molecules. 2022 Sep 9;27(18):5857. doi: 10.3390/molecules27185857. Molecules. 2022. PMID: 36144594 Free PMC article. Review.
-
Shellfish Allergy: a Comprehensive Review.Clin Rev Allergy Immunol. 2015 Oct;49(2):203-16. doi: 10.1007/s12016-014-8429-8. Clin Rev Allergy Immunol. 2015. PMID: 24870065 Review.
References
-
- Ellman LK, Chatchatee P, Sicherer SH, Sampson HA. Food hypersensitivity in two groups of children and young adults with atopic dermatitis evaluated a decade apart. Pediatr. Allergy. Immunol. 2002;13:295–298. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy. J. Allergy. Clin. Immunol. 2010;125:S116–125. - PubMed
-
- Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PV. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J. Immunol. 1993;151:5354–5363. - PubMed
-
- Jeoung BJ, Reese G, Hauck P, Oliver JB, Daul CB, Lehrer SB. Quantification of the major brown shrimp allergen Pen a 1 (tropomyosin) by a monoclonal antibody-based sandwich ELISA. J. Allergy. Clin. Immunol. 1997;100:229–234. - PubMed
-
- Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan-allergen. Int. Arch. Allergy. Immunol. 1999;119:247–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

