Scalable, stereocontrolled total syntheses of (±)-axinellamines A and B
- PMID: 21846138
- PMCID: PMC3164937
- DOI: 10.1021/ja206191g
Scalable, stereocontrolled total syntheses of (±)-axinellamines A and B
Abstract
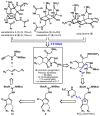
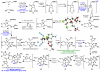
The development of a simple, efficient, scalable, and stereocontrolled synthesis of a common intermediate en route to the axinellamines, massadines, and palau'amine is reported. This completely new route was utilized to prepare the axinellamines on a gram scale. In a more general sense, three distinct and enabling methodological advances were made during these studies: (1) an ethylene glycol-assisted Pauson-Khand cycloaddition reaction, (2) a Zn/In-mediated Barbier-type reaction, and (3) a TfNH(2)-assisted chlorination-spirocyclization.
Figures
References
-
-
Urban S, Leone P. d. A., Carroll AR, Fechner GA, Smith J, Hooper JNA, Quinn RJ. J. Org. Chem. 1999;64:731–735. Nishimura S, Matsunaga S, Shibazaki M, Suzuki K, Furihata K, Van SRWM, Fusetani N. Org. Lett. 2003;5:2255–2257. Grube A, Immel S, Baran PS, Koeck M. Angew. Chem., Int. Ed. 2007;46:6721–6724. Kinnel RB, Gehrken HP, Scheuer PJ. J. Am. Chem. Soc. 1993;115:3376–3377. Kinnel RB, Gehrken H-P, Swali R, Skoropowski G, Scheuer PJ. J. Org. Chem. 1998;63:3281–3286. For a review, see: Köck M, Grube A, Seiple IB, Baran PS. Angew. Chem., Int. Ed. 2007;46:6586–6594.
-
-
-
For a comprehensive listing of studies towards the PIA family, see reference and Seiple IB, Su S, Young IS, Lewis CA, Yamaguchi J, Baran PS. Angew. Chem., Int. Ed. 2010;49:1095–1098. For recent studies, see Namba K, Inai M, Sundermeier U, Greshock TJ, Williams RM. Tetrahedron Lett. 2010;51:6557–6559. Feldman KS, Nuriye AY, Li J. J. Org. Chem. 2011;76:5042–5060. Chinigo GM, Breder A, Carreira EM. Org. Lett. 2011;13:78–81. Ma Z, Lu J, Wang X, Chen C. Chem. Commun. 2011;47:427–429.
-
-
- O’Malley DP, Yamaguchi J, Young IS, Seiple IB, Baran PS. Angew. Chem., Int. Ed. 2008;47:3581–3583. - PubMed
- Yamaguchi J, Seiple IB, Young IS, O’Malley DP, Maue M, Baran PS. Angew. Chem., Int. Ed. 2008;47:3578–3580. - PubMed
- Su S, Seiple IB, Young IS, Baran PS. J. Am. Chem. Soc. 2008;130:16490–16491. - PMC - PubMed
-
- Gaich T, Baran PS. J. Org. Chem. 2010;75:4657–4673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

