Liver-specific inducible nitric-oxide synthase expression is sufficient to cause hepatic insulin resistance and mild hyperglycemia in mice
- PMID: 21846719
- PMCID: PMC3186386
- DOI: 10.1074/jbc.M110.187666
Liver-specific inducible nitric-oxide synthase expression is sufficient to cause hepatic insulin resistance and mild hyperglycemia in mice
Erratum in
- J Biol Chem. 2011 Nov 11;286(45):39673. Shiota, Goshi [added]
Abstract
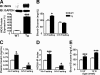
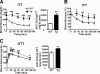
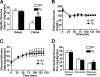
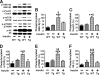
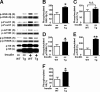
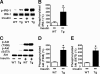
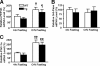
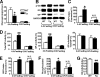
Inducible nitric-oxide synthase (iNOS), a major mediator of inflammation, plays an important role in obesity-induced insulin resistance. Inhibition of iNOS by gene disruption or pharmacological inhibitors reverses or ameliorates obesity-induced insulin resistance in skeletal muscle and liver in mice. It is unknown, however, whether increased expression of iNOS is sufficient to cause insulin resistance in vivo. To address this issue, we generated liver-specific iNOS transgenic (L-iNOS-Tg) mice, where expression of the transgene, iNOS, is regulated under mouse albumin promoter. L-iNOS-Tg mice exhibited mild hyperglycemia, hyperinsulinemia, insulin resistance, and impaired insulin-induced suppression of hepatic glucose output, as compared with wild type (WT) littermates. Insulin-stimulated phosphorylation of insulin receptor substrate-1 (IRS-1) and -2, and Akt was significantly attenuated in liver, but not in skeletal muscle, of L-iNOS-Tg mice relative to WT mice without changes in insulin receptor phosphorylation. Moreover, liver-specific iNOS expression abrogated insulin-stimulated phosphorylation of glycogen synthase kinase-3β, forkhead box O1, and mTOR (mammalian target of rapamycin), endogenous substrates of Akt, along with increased S-nitrosylation of Akt relative to WT mice. However, the expression of insulin receptor, IRS-1, IRS-2, Akt, glycogen synthase kinase-3β, forkhead box O1, protein-tyrosine phosphatase-1B, PTEN (phosphatase and tensin homolog), and p85 phosphatidylinositol 3-kinase was not altered by iNOS transgene. Hyperglycemia was associated with elevated glycogen phosphorylase activity and decreased glycogen synthase activity in the liver of L-iNOS-Tg mice, whereas phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, and proliferator-activated receptor γ coactivator-1α expression were not altered. These results clearly indicate that selective expression of iNOS in liver causes hepatic insulin resistance along with deranged insulin signaling, leading to hyperglycemia and hyperinsulinemia. Our data highlight a critical role for iNOS in the development of hepatic insulin resistance and hyperglycemia.
Figures







Similar articles
-
Inducible nitric oxide synthase deficiency ameliorates skeletal muscle insulin resistance but does not alter unexpected lower blood glucose levels after burn injury in C57BL/6 mice.Metabolism. 2012 Jan;61(1):127-36. doi: 10.1016/j.metabol.2011.06.001. Epub 2011 Aug 3. Metabolism. 2012. PMID: 21816442 Free PMC article.
-
Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins.Diabetes. 2010 Apr;59(4):861-71. doi: 10.2337/db09-1238. Epub 2010 Jan 26. Diabetes. 2010. PMID: 20103705 Free PMC article.
-
A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice.Diabetes. 2005 May;54(5):1340-8. doi: 10.2337/diabetes.54.5.1340. Diabetes. 2005. PMID: 15855318
-
Nitrosative stress and pathogenesis of insulin resistance.Antioxid Redox Signal. 2007 Mar;9(3):319-29. doi: 10.1089/ars.2006.1464. Antioxid Redox Signal. 2007. PMID: 17184170 Review.
-
Approaches to Decrease Hyperglycemia by Targeting Impaired Hepatic Glucose Homeostasis Using Medicinal Plants.Front Pharmacol. 2021 Dec 23;12:809994. doi: 10.3389/fphar.2021.809994. eCollection 2021. Front Pharmacol. 2021. PMID: 35002743 Free PMC article. Review.
Cited by
-
iNOS promotes hypothalamic insulin resistance associated with deregulation of energy balance and obesity in rodents.Sci Rep. 2017 Aug 23;7(1):9265. doi: 10.1038/s41598-017-08920-z. Sci Rep. 2017. PMID: 28835706 Free PMC article.
-
The Role of Insulin Resistance in Fueling NAFLD Pathogenesis: From Molecular Mechanisms to Clinical Implications.J Clin Med. 2022 Jun 24;11(13):3649. doi: 10.3390/jcm11133649. J Clin Med. 2022. PMID: 35806934 Free PMC article. Review.
-
Resilient hepatic mitochondrial function and lack of iNOS dependence in diet-induced insulin resistance.PLoS One. 2019 Feb 4;14(2):e0211733. doi: 10.1371/journal.pone.0211733. eCollection 2019. PLoS One. 2019. PMID: 30716103 Free PMC article.
-
iNOS as a Driver of Inflammation and Apoptosis in Mouse Skeletal Muscle after Burn Injury: Possible Involvement of Sirt1 S-Nitrosylation-Mediated Acetylation of p65 NF-κB and p53.PLoS One. 2017 Jan 18;12(1):e0170391. doi: 10.1371/journal.pone.0170391. eCollection 2017. PLoS One. 2017. PMID: 28099528 Free PMC article.
-
Hepatic Lysosomal iNOS Activity Impairs Autophagy in Obesity.Cell Mol Gastroenterol Hepatol. 2019;8(1):95-110. doi: 10.1016/j.jcmgh.2019.03.005. Epub 2019 Mar 27. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30926581 Free PMC article.
References
-
- Kaneki M., Shimizu N., Yamada D., Chang K. (2007) Antioxid. Redox. Signal 9, 319–329 - PubMed
-
- Arkan M. C., Hevener A. L., Greten F. R., Maeda S., Li Z. W., Long J. M., Wynshaw-Boris A., Poli G., Olefsky J., Karin M. (2005) Nat. Med. 11, 191–198 - PubMed
-
- Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) Nature 420, 333–336 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

