Mammalian ACSF3 protein is a malonyl-CoA synthetase that supplies the chain extender units for mitochondrial fatty acid synthesis
- PMID: 21846720
- PMCID: PMC3190830
- DOI: 10.1074/jbc.M111.291591
Mammalian ACSF3 protein is a malonyl-CoA synthetase that supplies the chain extender units for mitochondrial fatty acid synthesis
Abstract
The objective of this study was to identify a source of intramitochondrial malonyl-CoA that could be used for de novo fatty acid synthesis in mammalian mitochondria. Because mammalian mitochondria lack an acetyl-CoA carboxylase capable of generating malonyl-CoA inside mitochondria, the possibility that malonate could act as a precursor was investigated. Although malonyl-CoA synthetases have not been identified previously in animals, interrogation of animal protein sequence databases identified candidates that exhibited sequence similarity to known prokaryotic forms. The human candidate protein ACSF3, which has a predicted N-terminal mitochondrial targeting sequence, was cloned, expressed, and characterized as a 65-kDa acyl-CoA synthetase with extremely high specificity for malonate and methylmalonate. An arginine residue implicated in malonate binding by prokaryotic malonyl-CoA synthetases was found to be positionally conserved in animal ACSF3 enzymes and essential for activity. Subcellular fractionation experiments with HEK293T cells confirmed that human ACSF3 is located exclusively in mitochondria, and RNA interference experiments verified that this enzyme is responsible for most, if not all, of the malonyl-CoA synthetase activity in the mitochondria of these cells. In conclusion, unlike fungi, which have an intramitochondrial acetyl-CoA carboxylase, animals require an alternative source of mitochondrial malonyl-CoA; the mitochondrial ACSF3 enzyme is capable of filling this role by utilizing free malonic acid as substrate.
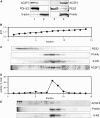
Figures



References
-
- Autio K. J., Kastaniotis A. J., Pospiech H., Miinalainen I. J., Schonauer M. S., Dieckmann C. L., Hiltunen J. K. (2008) FASEB J. 22, 569–578 - PubMed
-
- Chen Z., Kastaniotis A. J., Miinalainen I. J., Rajaram V., Wierenga R. K., Hiltunen J. K. (2009) FASEB J. 23, 3682–3691 - PubMed
-
- Miinalainen I. J., Chen Z. J., Torkko J. M., Pirilä P. L., Sormunen R. T., Bergmann U., Qin Y. M., Hiltunen J. K. (2003) J. Biol. Chem. 278, 20154–20161 - PubMed
-
- Zhang L., Joshi A. K., Hofmann J., Schweizer E., Smith S. (2005) J. Biol. Chem. 280, 12422–12429 - PubMed
-
- Zhang L., Joshi A. K., Smith S. (2003) J. Biol. Chem. 278, 40067–40074 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

