Lysosomal storage causes cellular dysfunction in mucolipidosis II skin fibroblasts
- PMID: 21846724
- PMCID: PMC3186395
- DOI: 10.1074/jbc.M111.267930
Lysosomal storage causes cellular dysfunction in mucolipidosis II skin fibroblasts
Abstract
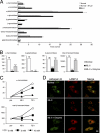
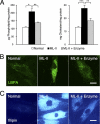
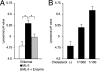
Mucolipidosis II (ML-II) is a fatal inherited metabolic disease caused by deficiency of GlcNAc-phosphotransferase, which plays a role in generating the mannose 6-phosphate recognition marker on lysosomal enzymes. In ML-II, many lysosomal acid hydrolases are mistargeted out of cells, and lysosomes become filled with undigested substrates, which explains inclusion cell disease as an alternative name for this disease. In this study, we revealed various cellular phenotypes in ML-II skin fibroblasts. We quantitated phospholipid and cholesterol within cells and showed ~2-fold accumulation in ML-II as compared with normal cells. Lysosomal pH of ML-II cells was higher than that of normal cells (5.29 ± 0.08 versus 4.79 ± 0.10, p < 0.001). The proliferated lysosomes in ML-II cells were accumulated ~3-fold in amount as compared with normal cells. Intracellular logistics including endocytosis and mannose 6-phosphate receptor recycling were impaired in ML-II cells. To confirm whether these ML-II cellular phenotypes derive from deficient lysosomal acid hydrolases within lysosomes, we performed supplementation of lysosomal enzymes using a partially purified total enzyme mixture, which was derived from the conditioned culture medium of normal skin fibroblasts after NH(4)Cl treatment. This supplementation corrected all of the previously described ML-II phenotypes. In addition, the autophagic and mitochondrial impairment that we have previously reported improved, and inclusion bodies disappeared on electron micrography following total lysosomal enzyme supplementation. Our results indicate that various cellular phenotypes in ML-II are caused by the deficiency of many lysosomal enzymes and massive accumulation of undigested substrates.
Figures




Similar articles
-
Lysosomal integral membrane glycoproteins are expressed at high levels in the inclusion bodies of I-cell disease fibroblasts.Arch Biochem Biophys. 1989 May 15;271(1):157-67. doi: 10.1016/0003-9861(89)90266-x. Arch Biochem Biophys. 1989. PMID: 2540710
-
Lysosomal Proteome and Secretome Analysis Identifies Missorted Enzymes and Their Nondegraded Substrates in Mucolipidosis III Mouse Cells.Mol Cell Proteomics. 2018 Aug;17(8):1612-1626. doi: 10.1074/mcp.RA118.000720. Epub 2018 May 17. Mol Cell Proteomics. 2018. PMID: 29773673 Free PMC article.
-
Analysis of mucolipidosis II/III GNPTAB missense mutations identifies domains of UDP-GlcNAc:lysosomal enzyme GlcNAc-1-phosphotransferase involved in catalytic function and lysosomal enzyme recognition.J Biol Chem. 2015 Jan 30;290(5):3045-56. doi: 10.1074/jbc.M114.612507. Epub 2014 Dec 11. J Biol Chem. 2015. PMID: 25505245 Free PMC article.
-
Mucolipidoses Overview: Past, Present, and Future.Int J Mol Sci. 2020 Sep 17;21(18):6812. doi: 10.3390/ijms21186812. Int J Mol Sci. 2020. PMID: 32957425 Free PMC article. Review.
-
Lysosomal Ca(2+) homeostasis: role in pathogenesis of lysosomal storage diseases.Cell Calcium. 2011 Aug;50(2):200-5. doi: 10.1016/j.ceca.2011.03.010. Epub 2011 Jul 2. Cell Calcium. 2011. PMID: 21724254 Review.
Cited by
-
Examining the Role of a Functional Deficiency of Iron in Lysosomal Storage Disorders with Translational Relevance to Alzheimer's Disease.Cells. 2023 Nov 16;12(22):2641. doi: 10.3390/cells12222641. Cells. 2023. PMID: 37998376 Free PMC article. Review.
-
Altered Met receptor phosphorylation and LRP1-mediated uptake in cells lacking carbohydrate-dependent lysosomal targeting.J Biol Chem. 2017 Sep 8;292(36):15094-15104. doi: 10.1074/jbc.M117.790139. Epub 2017 Jul 19. J Biol Chem. 2017. PMID: 28724630 Free PMC article.
-
Triclabendazole suppresses cellular levels of glycosaminoglycan-A potential therapeutic agent for mucopolysaccharidoses and related diseases.iScience. 2025 Jul 18;28(8):113118. doi: 10.1016/j.isci.2025.113118. eCollection 2025 Aug 15. iScience. 2025. PMID: 40822349 Free PMC article.
-
Lipophagy and Lipolysis Status in Lipid Storage and Lipid Metabolism Diseases.Int J Mol Sci. 2020 Aug 25;21(17):6113. doi: 10.3390/ijms21176113. Int J Mol Sci. 2020. PMID: 32854299 Free PMC article. Review.
-
Inhibition of lysosomal Ca2+ signalling disrupts dendritic spine structure and impairs wound healing in neurons.Commun Integr Biol. 2017 Nov 3;10(5-6):e1344802. doi: 10.1080/19420889.2017.1344802. eCollection 2017. Commun Integr Biol. 2017. PMID: 29259727 Free PMC article.
References
-
- Kornfeld S., Sly W. S. (2001) in The Metabolic and Molecular Bases of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D. eds) pp. 3421–3452, 8th Ed., McGraw-Hill, New York
-
- Storch S., Braulke T. (2005) in Lysosomes (Saftig P. ed) pp. 17–26, 1st Ed., Eurekah, Landes Bioscience, Springer and Business Media, New York
-
- Kornfeld R., Bao M., Brewer K., Noll C., Canfield W. (1999) J. Biol. Chem. 274, 32778–32785 - PubMed
-
- Kudo M., Bao M., D'Souza A., Ying F., Pan H., Roe B. A., Canfield W. M. (2005) J. Biol. Chem. 280, 36141–36149 - PubMed
-
- Tiede S., Storch S., Lübke T., Henrissat B., Bargal R., Raas-Rothschild A., Braulke T. (2005) Nat. Med. 11, 1109–1112 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

