Evidence that diacylglycerol acyltransferase 1 (DGAT1) has dual membrane topology in the endoplasmic reticulum of HepG2 cells
- PMID: 21846726
- PMCID: PMC3196132
- DOI: 10.1074/jbc.M111.251900
Evidence that diacylglycerol acyltransferase 1 (DGAT1) has dual membrane topology in the endoplasmic reticulum of HepG2 cells
Abstract
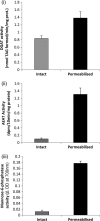
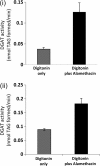
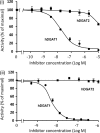
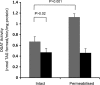
Triacylglycerol (TAG) synthesis and secretion are important functions of the liver that have major impacts on health, as overaccumulation of TAG within the liver (steatosis) or hypersecretion of TAG within very low density lipoproteins (VLDL) both have deleterious metabolic consequences. Two diacylglycerol acyltransferases (DGATs 1 and 2) can catalyze the final step in the synthesis of TAG from diacylglycerol, which has been suggested to play an important role in the transfer of the glyceride moiety across the endoplasmic reticular membrane for (re)synthesis of TAG on the lumenal aspect of the endoplasmic reticular (ER) membrane (Owen, M., Corstorphine, C. C., and Zammit, V. A. (1997) Biochem. J. 323, 17-21). Recent topographical studies suggested that the oligomeric enzyme DGAT1 is exclusively lumen facing (latent) in the ER membrane. By contrast, in the present study, using two specific inhibitors of human DGAT1, we present evidence that DGAT1 has a dual topology within the ER of HepG2 cells, with approximately equal DGAT1 activities exposed on the cytosolic and lumenal aspects of the ER membrane. This was confirmed by the observation of the loss of both overt (partial) and latent (total) DGAT activity in microsomes prepared from livers of Dgat1(-/-) mice. Conformational differences between DGAT1 molecules having the different topologies were indicated by the markedly disparate sensitivities of the overt DGAT1 to one of the inhibitors. These data suggest that DGAT1 belongs to the family of oligomeric membrane proteins that adopt a dual membrane topology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

