Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy
- PMID: 21846776
- PMCID: PMC3241659
- DOI: 10.1093/nar/gkr654
Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy
Abstract
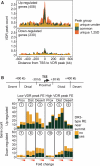
A global understanding of the actions of the nuclear hormone 1α,25-dihydroxyvitamin D(3) (1α,25(OH)(2)D(3)) and its vitamin D receptor (VDR) requires a genome-wide analysis of VDR binding sites. In THP-1 human monocytic leukemia cells we identified by ChIP-seq 2340 VDR binding locations, of which 1171 and 520 occurred uniquely with and without 1α,25(OH)(2)D(3) treatment, respectively, while 649 were common. De novo identified direct repeat spaced by 3 nucleotides (DR3)-type response elements (REs) were strongly associated with the ligand-responsiveness of VDR occupation. Only 20% of the VDR peaks diminishing most after ligand treatment have a DR3-type RE, in contrast to 90% for the most growing peaks. Ligand treatment revealed 638 1α,25(OH)(2)D(3) target genes enriched in gene ontology categories associated with immunity and signaling. From the 408 upregulated genes, 72% showed VDR binding within 400 kb of their transcription start sites (TSSs), while this applied only for 43% of the 230 downregulated genes. The VDR loci showed considerable variation in gene regulatory scenarios ranging from a single VDR location near the target gene TSS to very complex clusters of multiple VDR locations and target genes. In conclusion, ligand binding shifts the locations of VDR occupation to DR3-type REs that surround its target genes and occur in a large variety of regulatory constellations.
Figures




References
-
- Carlberg C, Polly P. Gene regulation by vitamin D3. Crit. Rev. Eukaryot. Gene Expr. 1998;8:19–42. - PubMed
-
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004;80:1689S–1696S. - PubMed
-
- Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr. Med. Res. Opin. 2008;24:139–149. - PubMed
-
- Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78:140–145. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

