Analysis of alternative splicing associated with aging and neurodegeneration in the human brain
- PMID: 21846794
- PMCID: PMC3202275
- DOI: 10.1101/gr.122226.111
Analysis of alternative splicing associated with aging and neurodegeneration in the human brain
Abstract
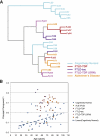
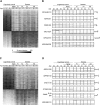
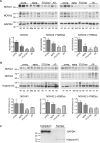
Age is the most important risk factor for neurodegeneration; however, the effects of aging and neurodegeneration on gene expression in the human brain have most often been studied separately. Here, we analyzed changes in transcript levels and alternative splicing in the temporal cortex of individuals of different ages who were cognitively normal, affected by frontotemporal lobar degeneration (FTLD), or affected by Alzheimer's disease (AD). We identified age-related splicing changes in cognitively normal individuals and found that these were present also in 95% of individuals with FTLD or AD, independent of their age. These changes were consistent with increased polypyrimidine tract binding protein (PTB)-dependent splicing activity. We also identified disease-specific splicing changes that were present in individuals with FTLD or AD, but not in cognitively normal individuals. These changes were consistent with the decreased neuro-oncological ventral antigen (NOVA)-dependent splicing regulation, and the decreased nuclear abundance of NOVA proteins. As expected, a dramatic down-regulation of neuronal genes was associated with disease, whereas a modest down-regulation of glial and neuronal genes was associated with aging. Whereas our data indicated that the age-related splicing changes are regulated independently of transcript-level changes, these two regulatory mechanisms affected expression of genes with similar functions, including metabolism and DNA repair. In conclusion, the alternative splicing changes identified in this study provide a new link between aging and neurodegeneration.
Figures

References
-
- Almeida A, Moncada S, Bolanos JP 2004. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6: 45–51 - PubMed
-
- Anthony K, Gallo JM 2010. Aberrant RNA processing events in neurological disorders. Brain Res 1338: 67–77 - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. 2006. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442: 916–919 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
