Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI
- PMID: 21846838
- PMCID: PMC3236630
- DOI: 10.1096/fj.11-185140
Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI
Abstract
A major limitation of tissue engineering research is the lack of noninvasive monitoring techniques for observations of dynamic changes in single tissue-engineered constructs. We use cellular magnetic resonance imaging (MRI) to track the fate of cells seeded onto functional tissue-engineered vascular grafts (TEVGs) through serial imaging. After in vitro optimization, murine macrophages were labeled with ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles and seeded onto scaffolds that were surgically implanted as inferior vena cava interposition grafts in SCID/bg mice. Serial MRI showed the transverse relaxation times (T(2)) were significantly lower immediately following implantation of USPIO-labeled scaffolds (T(2) = 44 ± 6.8 vs. 71 ± 10.2 ms) but increased rapidly at 2 h to values identical to control implants seeded with unlabeled macrophages (T(2) = 63 ± 12 vs. 63 ± 14 ms). This strongly indicates the rapid loss of seeded cells from the scaffolds, a finding verified using Prussian blue staining for iron containing macrophages on explanted TEVGs. Our results support a novel paradigm where seeded cells are rapidly lost from implanted scaffolds instead of developing into cells of the neovessel, as traditionally thought. Our findings confirm and validate this paradigm shift while demonstrating the first successful application of noninvasive MRI for serial study of cellular-level processes in tissue engineering.
Figures


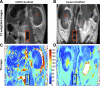
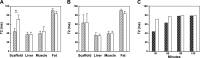

Similar articles
-
Initial evaluation of the use of USPIO cell labeling and noninvasive MR monitoring of human tissue-engineered vascular grafts in vivo.FASEB J. 2008 Nov;22(11):3888-95. doi: 10.1096/fj.08-107367. Epub 2008 Aug 18. FASEB J. 2008. PMID: 18711027 Free PMC article.
-
Targeted imaging of matrix metalloproteinase activity in the evaluation of remodeling tissue-engineered vascular grafts implanted in a growing lamb model.J Thorac Cardiovasc Surg. 2014 Nov;148(5):2227-33. doi: 10.1016/j.jtcvs.2014.05.037. Epub 2014 May 21. J Thorac Cardiovasc Surg. 2014. PMID: 24952823 Free PMC article.
-
Tissue-engineered vascular grafts: does cell seeding matter?J Pediatr Surg. 2010 Jun;45(6):1299-305. doi: 10.1016/j.jpedsurg.2010.02.102. J Pediatr Surg. 2010. PMID: 20620335 Free PMC article.
-
The Current Status of Tissue-Engineered Vascular Grafts.Cardiol Rev. 2015 Sep-Oct;23(5):236-9. doi: 10.1097/CRD.0000000000000060. Cardiol Rev. 2015. PMID: 25699981 Review.
-
Electrospinning of biomimetic scaffolds for tissue-engineered vascular grafts: threading the path.Expert Rev Cardiovasc Ther. 2014 Jul;12(7):815-32. doi: 10.1586/14779072.2014.925397. Epub 2014 Jun 6. Expert Rev Cardiovasc Ther. 2014. PMID: 24903895 Review.
Cited by
-
Biomaterial-driven in situ cardiovascular tissue engineering-a multi-disciplinary perspective.NPJ Regen Med. 2017 Jun 16;2:18. doi: 10.1038/s41536-017-0023-2. eCollection 2017. NPJ Regen Med. 2017. PMID: 29302354 Free PMC article. Review.
-
Magnetic Resonance Imaging of Shear Stress and Wall Thickness in Tissue-Engineered Vascular Grafts.Tissue Eng Part C Methods. 2018 Aug;24(8):465-473. doi: 10.1089/ten.TEC.2018.0144. Epub 2018 Jul 31. Tissue Eng Part C Methods. 2018. PMID: 29978768 Free PMC article.
-
Application of medical imaging methods and artificial intelligence in tissue engineering and organ-on-a-chip.Front Bioeng Biotechnol. 2022 Sep 12;10:985692. doi: 10.3389/fbioe.2022.985692. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36172022 Free PMC article. Review.
-
Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering.Nanomaterials (Basel). 2021 Sep 8;11(9):2337. doi: 10.3390/nano11092337. Nanomaterials (Basel). 2021. PMID: 34578651 Free PMC article. Review.
-
Deconstructing the Tissue Engineered Vascular Graft: Evaluating Scaffold Pre-Wetting, Conditioned Media Incubation, and Determining the Optimal Mononuclear Cell Source.ACS Biomater Sci Eng. 2017;3(9):1972-1979. doi: 10.1021/acsbiomaterials.6b00123. Epub 2016 Aug 8. ACS Biomater Sci Eng. 2017. PMID: 29226239 Free PMC article.
References
-
- Kim S.-J., Kim W.-H., Lim H.-G., Lee J.-Y. (2008) Outcome of 200 patients after an extracardiac Fontan procedure. J. Thoracic Cardiovasc. Surg. 136, 108–116 - PubMed
-
- Gentles T. L., Gauvreau K., Mayer J. E., Fishberger S. B., Burnett J., Colan S. D., Newburger J. W., Wernovsky G. (1997) Functional outcome after the Fontan operation: factors influencing late morbidity. J. Thorac. Cardiovasc. Surg. 114, 392–403 - PubMed
-
- Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., Shinoka T. (2010) Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 139, 431–436 - PubMed
-
- Shin'oka T., Matsumura G., Hibino N., Naito Y., Watanabe M., Konuma T., Sakamoto T, Nagatsu M., Kurosawa H. (2005) Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 129, 1330–1338 - PubMed
-
- Roh J. D., Nelson G. N., Brennan M. P., Mirensky T. L., Yi T., Hazlett T. F., Tellides G., Sinusas A. J., Pober J. S., Saltzman W. M., Kyriakides T. R., Breuer C. K. (2008) Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 29, 1454–1463 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

