Synthesis and contractile activity of substituted 1,2,3,4-tetrahydroisoquinolines
- PMID: 21847072
- PMCID: PMC6264338
- DOI: 10.3390/molecules16087019
Synthesis and contractile activity of substituted 1,2,3,4-tetrahydroisoquinolines
Abstract
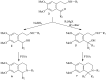
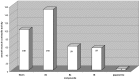
A series of different 1-monosubstituted and 1,1-disubstituted 1,2,3,4-tetrahydro-isoquinolines was synthesized in high yields from different ketoamides. We have developed a convenient method for the synthesis of disubstituted derivatives by interaction of ketoamides with organomagnesium compounds, followed by cyclization in the presence of catalytic amounts of p-toluenesulfonic acid (PTSA). A number of substituents at the C-1 in the isoquinoline skeleton were introduced varying either carboxylic acid or organomagnesium compound. Some of the obtained 1,1-dialkyl-1,2,3,4-tetrahydro-isoquinolines possess contractile activity against guinea pig's gastric smooth muscle preparations.
Figures





Similar articles
-
Correlation between the hydrolysis constants and spasmolytic activities of some isoquinolines.Pharmazie. 1977 Nov;32(11):720-1. Pharmazie. 1977. PMID: 609595
-
Retigabine diminishes the effects of acetylcholine, adrenaline and adrenergic agonists on the spontaneous activity of guinea pig smooth muscle strips in vitro.Auton Neurosci. 2017 Mar;203:51-57. doi: 10.1016/j.autneu.2016.12.006. Epub 2016 Dec 23. Auton Neurosci. 2017. PMID: 28041987
-
[Study on the response characteristics of the in vivo bladder detrusor to the cholinergic transmitter].Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2008 Aug;24(3):360-2. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2008. PMID: 21141604 Chinese. No abstract available.
-
[Isolation, configuration and contractile responses of single smooth muscle cells].Nihon Yakurigaku Zasshi. 1993 Mar;101(3):101-10. doi: 10.1254/fpj.101.3_101. Nihon Yakurigaku Zasshi. 1993. PMID: 8486318 Review. Japanese.
-
Muscarinic acetylcholine receptors in smooth muscles: regulation of contraction and molecular nature.Adv Biochem Psychopharmacol. 1983;36:31-41. Adv Biochem Psychopharmacol. 1983. PMID: 6305155 Review. No abstract available.
Cited by
-
Therapeutic Potential of 1-(2-Chlorophenyl)-6,7-dimethoxy-3-methyl-3,4-dihydroisoquinoline.Molecules. 2024 Aug 11;29(16):3804. doi: 10.3390/molecules29163804. Molecules. 2024. PMID: 39202883 Free PMC article.
-
Synthesis of N-[1-(2-Acetyl-4,5-dimethoxyphenyl)propan-2-yl]benzamide and Its Copper(II) Complex.Russ J Gen Chem. 2023;93(1):161-165. doi: 10.1134/S1070363223010218. Epub 2023 Mar 9. Russ J Gen Chem. 2023. PMID: 36919098 Free PMC article.
-
Synthesis, Molecular Docking, and Biological Evaluation of Novel Anthranilic Acid Hybrid and Its Diamides as Antispasmodics.Int J Mol Sci. 2023 Sep 8;24(18):13855. doi: 10.3390/ijms241813855. Int J Mol Sci. 2023. PMID: 37762158 Free PMC article.
-
Experimental and theoretical perspectives of the Noyori-Ikariya asymmetric transfer hydrogenation of imines.Molecules. 2014 May 28;19(6):6987-7007. doi: 10.3390/molecules19066987. Molecules. 2014. PMID: 24879612 Free PMC article. Review.
-
A chiral HPLC and pharmacokinetic approach of 1-(4-bromophenyl)-6,7-dimethoxy-3,4-dihydroisoquinoline-2(1H)-sulfonamide.Bioanalysis. 2025 Apr;17(7):445-453. doi: 10.1080/17576180.2025.2481023. Epub 2025 Mar 24. Bioanalysis. 2025. PMID: 40126089
References
-
- Lundstrom J. In: The Alkaloids. Brossi A., editor. Vol. 21. Academic Press; New York, NY, USA: 1983. p. 255.
-
- Collins M.A. In: The Alkaloids. Brossi A., editor. Vol. 21. Academic Press; New York, NY, USA: 1983. p. 329.
-
- Chrzanowska M., Schönenberg B., Brossi A., Flippen-Anderson J.L. Mammalian alkaloids: Configurations of optically active salsoline- and 3′,4′-Dideoxynorlaudanosoline-1-carboxylic acids. Helv. Chim. Acta. 1987;70:1721–1731. doi: 10.1002/hlca.19870700707. - DOI
-
- Czarnocki Z., Suh D., MacLean D.B., Hultin P.G., Szarek W.A. Enantioselective synthesis of 1-substituted tetrahydroisoquinoline-1-carboxylic acids. Can. J. Chem. 1992;70:1555–1561. doi: 10.1139/v92-191. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

