Erythropoietin gene-enhanced marrow mesenchymal stromal cells decrease cisplatin-induced kidney injury and improve survival of allogeneic mice
- PMID: 21847101
- PMCID: PMC3222527
- DOI: 10.1038/mt.2011.162
Erythropoietin gene-enhanced marrow mesenchymal stromal cells decrease cisplatin-induced kidney injury and improve survival of allogeneic mice
Abstract
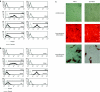
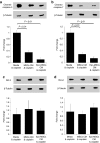
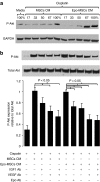
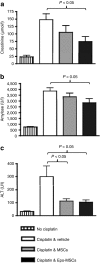
Bone marrow-derived mesenchymal stromal cells (MSCs) are promising for regenerative medicine applications, such as for renoprotection and repair in acute kidney injury (AKI). Erythropoietin (Epo) can also exert cytoprotective effects on various tissues including the kidney. We hypothesized that MSCs gene-enhanced to secrete Epo may produce a significant beneficial effect in AKI. Mouse Epo-secreting MSCs were generated, tested in vitro, and then implanted by intraperitoneal injection in allogeneic mice previously administered cisplatin to induce AKI. Epo-MSCs significantly improved survival of implanted mice as compared to controls (67% survival versus 33% with Vehicle only). Also, Epo-MSCs led to significantly better kidney function as shown by lower levels of blood urea nitrogen (72 ± 9.5 mg/dl versus 131 ± 9.20 mg/dl) and creatinine (74 ± 17 µmol/l versus 148±19.4 µmol/l). Recipient mice also showed significantly decreased amylase and alanine aminotransferase blood concentrations. Kidney sections revealed significantly less apoptotic cells and more proliferating cells. Furthermore, PCR revealed the presence of implanted cells in recipient kidneys, with Epo-MSCs leading to significantly increased expression of Epo and of phosphorylated-Akt (Ser473) (P-Akt) in these kidneys. In conclusion, our study demonstrates that Epo gene-enhanced MSCs exert significant tissue protective effects in allogeneic mice with AKI, and supports the potential use of gene-enhanced cells as universal donors in acute injury.
Figures




Similar articles
-
The Beneficial Effects of Mesenchymal Stem Cells in Acute Kidney Injury: A Narrative Review.Curr Stem Cell Res Ther. 2024;19(2):200-209. doi: 10.2174/1574888X18666230206115046. Curr Stem Cell Res Ther. 2024. PMID: 36748221 Free PMC article. Review.
-
Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection.Am J Physiol Renal Physiol. 2010 Dec;299(6):F1288-98. doi: 10.1152/ajprenal.00671.2009. Epub 2010 Sep 15. Am J Physiol Renal Physiol. 2010. PMID: 20844023
-
Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment.Exp Cell Res. 2013 Aug 1;319(13):2019-2027. doi: 10.1016/j.yexcr.2013.04.008. Epub 2013 Apr 24. Exp Cell Res. 2013. PMID: 23624354
-
Bone marrow-derived mesenchymal stem cells protect against cisplatin-induced acute kidney injury in rats by inhibiting cell apoptosis.Int J Mol Med. 2013 Dec;32(6):1262-72. doi: 10.3892/ijmm.2013.1517. Epub 2013 Oct 8. Int J Mol Med. 2013. PMID: 24126885 Free PMC article.
-
Immunomodulatory Effects of Mesenchymal Stem Cells on Drug-Induced Acute Kidney Injury.Front Immunol. 2021 Jun 4;12:683003. doi: 10.3389/fimmu.2021.683003. eCollection 2021. Front Immunol. 2021. PMID: 34149721 Free PMC article. Review.
Cited by
-
MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization.Stem Cell Res Ther. 2022 Apr 27;13(1):171. doi: 10.1186/s13287-022-02855-7. Stem Cell Res Ther. 2022. PMID: 35477552 Free PMC article.
-
(Mesenchymal) Stem Cell-Based Therapy in Cisplatin-Induced Acute Kidney Injury Animal Model: Risk of Immunogenicity and Tumorigenicity.Stem Cells Int. 2017;2017:7304643. doi: 10.1155/2017/7304643. Epub 2017 Dec 12. Stem Cells Int. 2017. PMID: 29379525 Free PMC article. Review.
-
Immunomodulatory plasticity of mesenchymal stem cells: a potential key to successful solid organ transplantation.J Transl Med. 2018 Feb 15;16(1):31. doi: 10.1186/s12967-018-1403-0. J Transl Med. 2018. PMID: 29448956 Free PMC article. Review.
-
The Beneficial Effects of Mesenchymal Stem Cells in Acute Kidney Injury: A Narrative Review.Curr Stem Cell Res Ther. 2024;19(2):200-209. doi: 10.2174/1574888X18666230206115046. Curr Stem Cell Res Ther. 2024. PMID: 36748221 Free PMC article. Review.
-
Mesenchymal Stem Cells as Therapeutic Agents and Novel Carriers for the Delivery of Candidate Genes in Acute Kidney Injury.Stem Cells Int. 2020 Dec 11;2020:8875554. doi: 10.1155/2020/8875554. eCollection 2020. Stem Cells Int. 2020. PMID: 33381189 Free PMC article. Review.
References
-
- Sagrinati C, Ronconi E, Lazzeri E, Lasagni L., and, Romagnani P. Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol Med. 2008;14:277–285. - PubMed
-
- Uccelli A, Moretta L., and, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. - PubMed
-
- Bi B, Schmitt R, Israilova M, Nishio H., and, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

