A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis
- PMID: 21847104
- PMCID: PMC3265373
- DOI: 10.1038/ncomms1444
A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis
Abstract
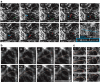
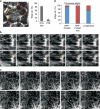
It is well known that the parallel order of microtubules in the plant cell cortex defines the direction of cell expansion, yet it remains unclear how microtubule orientation is controlled, especially on a cell-wide basis. Here we show through 4D imaging and computational modelling that plant cell polyhedral geometry provides spatial input that determines array orientation and heterogeneity. Microtubules depolymerize when encountering sharp cell edges head-on, whereas those oriented parallel to those sharp edges remain. Edge-induced microtubule depolymerization, however, is overcome by the microtubule-associated protein CLASP, which accumulates at specific cell edges, enables microtubule growth around sharp edges and promotes formation of microtubule bundles that span adjacent cell faces. By computationally modelling dynamic 'microtubules on a cube' with edges differentially permissive to microtubule passage, we show that the CLASP-edge complex is a 'tuneable' microtubule organizer, with the inherent flexibility to generate the numerous cortical array patterns observed in nature.
Figures





References
-
- Flanders D. J., Rawlins D. J., Shaw P. J. & Lloyd C. W. Computer-Aided 3-D Reconstruction of Interphase Microtubules in Epidermal-Cells of Datura-Stramonium Reveals Principles of Array Assembly. Development 106, 531–541 (1989).
-
- Shaw S. L., Kamyar R. & Ehrhardt D. W. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 300, 1715–1718 (2003). - PubMed
-
- Wasteneys G. O. & Collings D. A. C. The cytoskeleton and co-ordination of directional expansion in a multicellular context: in The Expanding Cell, Vol 5 (eds Verbelen, J.P. & Vissenberg, K.) 217–248 (Springer-Verlag, 2007).
-
- Ehrhardt D. W. Straighten up and fly right: microtubule dynamics and organization of non-centrosomal arrays in higher plants. Curr. Opin. Cell Biol. 20, 107–116 (2008). - PubMed
-
- Wasteneys G. O. & Ambrose J. C. Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends Cell Biol. 19, 62–71 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

