Gut microbiota: next frontier in understanding human health and development of biotherapeutics
- PMID: 21847343
- PMCID: PMC3156250
- DOI: 10.2147/BTT.S19099
Gut microbiota: next frontier in understanding human health and development of biotherapeutics
Abstract
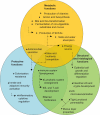
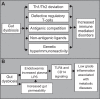
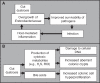
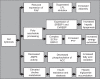
The gut microbiota is a remarkable asset for human health. As a key element in the development and prevention of specific diseases, its study has yielded a new field of promising biotherapeutics. This review provides comprehensive and updated knowledge of the human gut microbiota, its implications in health and disease, and the potentials and limitations of its modification by currently available biotherapeutics to treat, prevent and/or restore human health, and future directions. Homeostasis of the gut microbiota maintains various functions which are vital to the maintenance of human health. Disruption of the intestinal ecosystem equilibrium (gut dysbiosis) is associated with a plethora of human diseases, including autoimmune and allergic diseases, colorectal cancer, metabolic diseases, and bacterial infections. Relevant underlying mechanisms by which specific intestinal bacteria populations might trigger the development of disease in susceptible hosts are being explored across the globe. Beneficial modulation of the gut microbiota using biotherapeutics, such as prebiotics, probiotics, and antibiotics, may favor health-promoting populations of bacteria and can be exploited in development of biotherapeutics. Other technologies, such as development of human gut models, bacterial screening, and delivery formulations eg, microencapsulated probiotics, may contribute significantly in the near future. Therefore, the human gut microbiota is a legitimate therapeutic target to treat and/or prevent various diseases. Development of a clear understanding of the technologies needed to exploit the gut microbiota is urgently required.
Keywords: biotherapeutics; dysbiosis; gut microbiota; human health; microencapsulation; probiotics.
Figures
References
-
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. - PubMed
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
-
- McConnell EL, Fadda HM, Basit AW. Gut instincts: Explorations in intestinal physiology and drug delivery. Int J Pharm. 2008;364:213–226. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

