The Roles of Glutathione Peroxidases during Embryo Development
- PMID: 21847368
- PMCID: PMC3148772
- DOI: 10.3389/fnmol.2011.00012
The Roles of Glutathione Peroxidases during Embryo Development
Abstract
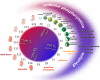
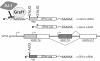
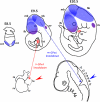
Embryo development relies on the complex interplay of the basic cellular processes including proliferation, differentiation, and apoptotic cell death. Precise regulation of these events is the basis for the establishment of embryonic structures and the organ development. Beginning with fertilization of the oocyte until delivery the developing embryo encounters changing environmental conditions such as varying levels of oxygen, which can give rise to reactive oxygen species (ROS). These challenges are met by the embryo with metabolic adaptations and by an array of anti-oxidative mechanisms. ROS can be deleterious by modifying biological molecules including lipids, proteins, and nucleic acids and may induce abnormal development or even embryonic lethality. On the other hand ROS are vital players of various signaling cascades that affect the balance between cell growth, differentiation, and death. An imbalance or dysregulation of these biological processes may generate cells with abnormal growth and is therefore potentially teratogenic and tumorigenic. Thus, a precise balance between processes generating ROS and those decomposing ROS is critical for normal embryo development. One tier of the cellular protective system against ROS constitutes the family of selenium-dependent glutathione peroxidases (GPx). These enzymes reduce hydroperoxides to the corresponding alcohols at the expense of reduced glutathione. Of special interest within this protein family is the moonlighting enzyme glutathione peroxidase 4 (Gpx4). This enzyme is a scavenger of lipophilic hydroperoxides on one hand, but on the other hand can be transformed into an enzymatically inactive cellular structural component. GPx4 deficiency - in contrast to all other GPx family members - leads to abnormal embryo development and finally produces a lethal phenotype in mice. This review is aimed at summarizing the current knowledge on GPx isoforms during embryo development and tumor development with an emphasis on GPx4.
Keywords: anti-oxidative defense; reactive oxygen species; selenium; teratogenesis.
Figures
References
-
- Arner E. S. (2009). Focus on mammalian thioredoxin reductases – important selenoproteins with versatile functions. Biochim. Biophys. Acta 1790, 495–526 - PubMed
-
- Avissar N., Eisenmann C., Breen J. G., Horowitz S., Miller R. K., Cohen H. J. (1994a). Human placenta makes extracellular glutathione peroxidase and secretes it into maternal circulation. Am. J. Physiol. 267, E68–E76 - PubMed
-
- Avissar N., Ornt D. B., Yagil Y., Horowitz S., Watkins R. H., Kerl E. A., Takahashi K., Palmer I. S., Cohen H. J. (1994b). Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am. J. Physiol. 266, C367–C375 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

