LDL from obese patients with the metabolic syndrome show increased lipid peroxidation and activate platelets
- PMID: 21847583
- PMCID: PMC3367234
- DOI: 10.1007/s00125-011-2272-8
LDL from obese patients with the metabolic syndrome show increased lipid peroxidation and activate platelets
Abstract
Aims/hypothesis: This study assessed oxidative stress in LDL from obese patients with the metabolic syndrome and compared it with that in LDL from type 2 diabetic patients or control volunteers. It also determined the effect on platelets of LDL from the three groups.
Methods: The profiles of lipids, fatty acids and fatty acid oxidation products were determined in LDL isolated from plasma of patients with the metabolic syndrome, patients with type 2 diabetes and volunteers (n = 10 per group). The effects of LDL from the participant groups on the platelet arachidonic acid signalling cascade and aggregation were investigated.
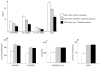
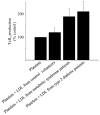
Results: Compared with LDL from control volunteers, LDL from obese metabolic syndrome and type 2 diabetic patients had lower cholesteryl ester, higher triacylglycerol and lower ethanolamine plasmalogen levels. Proportions of linoleic acid were decreased in phosphatidylcholine and cholesteryl esters in LDL from both patient groups. Among the markers of lipid peroxidation, oxidation products of linoleic acid (hydroxy-octadecadienoic acids) and malondialdehyde were increased by 59% and twofold, respectively in LDL from metabolic syndrome and type 2 diabetic patients. LDL from metabolic syndrome and type 2 diabetic patients were equally potent in activating the platelet arachidonic acid signalling cascade through increased phosphorylation of p38 mitogen-activated protein kinase and cytosolic phospholipase A(2), and through increased thromboxane B(2) formation. LDL from patients with the metabolic syndrome and type 2 diabetes potentiated platelet aggregation by threefold and 3.5-fold respectively, whereas control LDL had no activating effects on platelets.
Conclusions/interpretation: The metabolic syndrome in obese patients, without or with diabetes, is associated with increased oxidative stress in LDL, which triggers platelet activation.
Trial registration: ClinicalTrials.gov NCT00932087.
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures
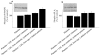

References
-
- Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202:321–329. - PubMed
-
- Hsu RM, Devaraj S, Jialal I. Autoantibodies to oxidized low-density lipoprotein in patients with type 2 diabetes mellitus. Clin Chim Acta. 2002;317:145–150. - PubMed
-
- Holvoet P, Kritchevsky SB, Tracy RP, et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53:1068–1073. - PubMed
-
- Sigurdardottir V, Fagerberg B, Hulthe J. Circulating oxidized low-density lipoprotein (LDL) is associated with risk factors of the metabolic syndrome and LDL size in clinically healthy 58-year-old men (AIR study) J Intern Med. 2002;252:440–447. - PubMed

